Pregnancy SmartSiteTM
Vaccines for COVID-19; COVID-19 vaccinations; COVID-19 shots; Vaccinations for COVID-19; COVID-19 immunizations; COVID-19 prevention - vaccines; mRNA vaccine - COVID-19; COVID-19 vaccine booster shots; Booster shots for COVID-19 DefinitionCOVID-19 vaccines are used to prepare the body's immune system to protect against COVID-19. Everyone ages 6 months and older should get a 2025-2026 COVID-19 vaccine. This includes pregnant women and those planning to become pregnant. You should get a 2025-2026 COVID-19 vaccine even if you:
InformationHOW COVID-19 VACCINES WORK COVID-19 vaccines protect people from getting COVID-19 and from getting more severe symptoms if they do get COVID-19. These vaccines teach your body how to defend against the SARS-CoV-2 virus, which causes COVID-19. COVID-19 vaccines have been shown to do a very good job of:
mRNA VACCINES The mRNA vaccines approved in the United States work differently from many other vaccines.
There are 3 mRNA COVID-19 vaccines currently approved for use in the United States:
The COVID-19 mRNA vaccine is given as an injection (shot) in the arm. The 2025-2026 mRNA vaccines protect against the current strains of the COVID-19 virus. SUBUNIT VACCINE The Novavax vaccine is a protein subunit vaccine. The vaccine includes harmless pieces of the spike protein of the SARS-CoV-2 virus. The vaccine triggers the body to develop antibodies to protect you from the virus. The 2025-2026 Novavax vaccine also protects against current strains of the COVID-19 virus. It is approved for use in people ages 12 years and older. VACCINATION SCHEDULE The vaccination schedule is based on:
The Centers for Disease Control and Prevention recommend that people of all ages consult their health care provider to decide if getting a COVID-19 vaccine is best for them. The provider may be a doctor, nurse, or pharmacist. This process is called shared decision-making. Most health experts recommend:
Adults and children who are severely immunocompromised may need additional doses of the 2025-2026 COVID-19 vaccine. Talk with your health care provider about whether you may need additional doses. People who recently had COVID-19 may delay getting a COVID-19 vaccine for 3 months. You are much less likely to get COVID-19 in the 3 months after having the illness. You may choose to get the vaccine sooner if you or someone in your family are at severe risk of illness or if there are high local rates of COVID-19. VACCINE MYTHS COVID-19 vaccines:
VACCINE SIDE EFFECTS While COVID-19 vaccines will not make you sick from COVID-19, they may cause certain side effects and flu-like symptoms. This is normal. These symptoms are a sign that your body is making antibodies against the virus. Side effects can vary from person to person. Common side effects include:
Some side effects from the shot may affect your ability to do daily activities, but any side effects will go away in a few days. Any side effects from the vaccine are far less dangerous than the potential for serious illness or death from COVID-19. HOW TO GET THE VACCINE There are several ways you can look for vaccination providers near you.
Learn what to expect when you get your COVID-19 vaccine. VACCINE SAFETY The safety of vaccines is the top priority, and COVID-19 vaccines have passed rigorous safety standards before approval. Millions of people have received the vaccine, and no long-term side effects have been reported. The vaccines continue to be closely monitored to ensure they are safe and effective. There have been reports of some people who have had an allergic reaction to the current vaccines. So it is important to follow certain precautions:
If you have had an allergic reaction, even if not severe, to other vaccines or injectable therapies, you should ask your provider if you should get a COVID-19 vaccine. Your provider will help you decide if it is safe to get vaccinated. Serious health events from COVID-19 vaccines, such as an allergic reaction, are rare. Adverse events after COVID-19 vaccination are very rare. Rare cases of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the outer lining of the heart) have been reported in children and teens ages 5 years and older after getting the COVID-19 vaccine. This reaction occurred more often in male adolescents and young adults ages 12 to 39 years. However, it has also occurred in females, in other age groups, after other doses, and after receiving any type of vaccine.
Symptoms of myocarditis and pericarditis include:
If your child or teenager has any of these symptoms, get medical help right away. All these associations are so rare that they should not cause hesitation in receiving any of these vaccines. The CDC recommends that people may still get vaccinated if they have a history of:
ReferencesAmerican Academy of Family Physicians website. COVID-19. www.aafp.org/family-physician/patient-care/public-health-emergencies/recent-outbreaks/covid-19.html. Accessed October 22, 2025. Centers for Disease Control and Prevention website. Vaccine safety: coronavirus disease 2019 (COVID-19) vaccine safety. www.cdc.gov/vaccine-safety/vaccines/covid-19.html. Updated January 31, 2025. Accessed October 22, 2025. Centers for Disease Control and Prevention website. COVID-19: benefits of getting vaccinated. www.cdc.gov/covid/vaccines/benefits.html. Updated June 11, 2025. Accessed October 22, 2025. Committee on Infectious Diseases. Recommendations for COVID-19 Vaccines in Infants, Children, and Adolescents: Policy Statement. Pediatrics. 2025 Aug 19. Epub ahead of print. PMID: 40826495. pubmed.ncbi.nlm.nih.gov/40826495/. Prasad V, Makary MA. An Evidence-Based Approach to Covid-19 Vaccination. N Engl J Med. 2025 Jun 26;392(24):2484-2486. Epub 2025 May 20. PMID: 40392534. pubmed.ncbi.nlm.nih.gov/40392534/. The American College of Obstetricians and Gynecologists website. COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care. www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care. Updated September 2025. Accessed October 22, 2025. U.S. Food and Drug Administration website. COMIRNATY. www.fda.gov/vaccines-blood-biologics/comirnaty. Updated September 25, 2025. Accessed October 22, 2025. U.S. Food and Drug Administration website. MNEXSPIKE. www.fda.gov/vaccines-blood-biologics/mnexspike. Updated September 25, 2025. Accessed October 22, 2025. U.S. Food and Drug Administration website. NUVAXOVID. www.fda.gov/vaccines-blood-biologics/vaccines/nuvaxovid. Updated September 25, 2025. Accessed October 22, 2025. U.S. Food and Drug Administration website. SPIKEVAX. www.fda.gov/vaccines-blood-biologics/spikevax. Updated September 25, 2025. Accessed October 22, 2025. U.S. Department of Health and Human Services. ACIP Recommends COVID-19 Immunization Based on Individual Decision-making. www.hhs.gov/press-room/acip-recommends-covid19-vaccination-individual-decision-making.html. Updated September 19, 2025. Accessed October 22, 2025. | ||
| ||
Review Date: 1/1/2025 Reviewed By: Linda J. Vorvick, MD, Clinical Professor Emeritus, Department of Family Medicine, UW Medicine, School of Medicine, University of Washington, Seattle, WA. Also reviewed by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team. Editorial update 10/23/2025. View References The information provided herein should not be used during any medical emergency or for the diagnosis or treatment of any medical condition. A licensed medical professional should be consulted for diagnosis and treatment of any and all medical conditions. Links to other sites are provided for information only -- they do not constitute endorsements of those other sites. No warranty of any kind, either expressed or implied, is made as to the accuracy, reliability, timeliness, or correctness of any translations made by a third-party service of the information provided herein into any other language. © 1997- A.D.A.M., a business unit of Ebix, Inc. Any duplication or distribution of the information contained herein is strictly prohibited. | ||


 COVID-19 vaccine
COVID-19 vaccine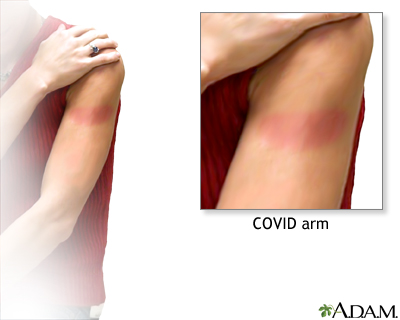 Arm Rash After COV...
Arm Rash After COV...
