Immunizations - InDepth
Highlights
Bacteria, viruses, parasites, and other germs (microbes) cause many human diseases. Vaccines can help protect against some of these diseases.
Data released recently shows that many immunization rates are below the Healthy People 2020 target rates. Vaccination (immunization) against infectious diseases saves millions of lives. Illness and death from diphtheria, pertussis, tetanus, measles, mumps, hepatitis, and other diseases can be prevented through immunization.
Introduction
Bacteria, viruses, parasites, and other germs (microbes) cause many human diseases. Vaccines can help protect against some of these diseases. Vaccination (immunization) against infectious diseases saves millions of lives. Illness and death from diphtheria, pertussis, tetanus, measles, mumps, hepatitis, and other diseases can be prevented through immunization.
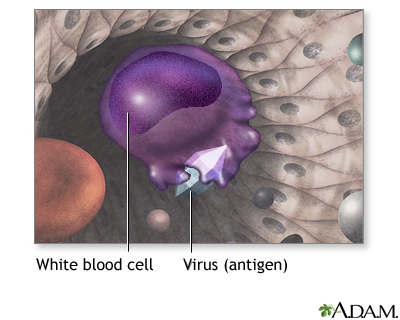
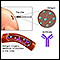
Your body is designed to protect you from infections. When you are exposed to a virus, bacterium, or other microbe, your immune system actually "learns" from the experience. The next time your body is exposed to the same microbe, your immune system often recognizes it and sets out to destroy it.
During immunization, you are exposed to an inactive/weakened microbe or a very small and safe amount of part of a microbe. Your body's immune system responds to the vaccine by making substances called antibodies. If you are exposed to the microbe itself at a later time, the antibodies will kill the microbe and prevent infection or you may have a milder infection.
Most vaccines are given by an injection. Some can be taken orally (by mouth) or by a nasal spray. Vaccines contain one of four components that trigger an immune response:
- Live but weakened virus. Live-virus vaccines provide longer immunity than inactivated ones. But they can cause serious infection in people with weakened immune systems. In rare cases, they have also been linked to other medical problems.
- Inactivated bacteria and viruses. Inactivated vaccines are safe even for people with weakened immune systems.
- Toxoid. This is an altered form of a harmful substance (toxin) released by certain bacteria. The toxoid in vaccines is changed so it is not harmful, but still produces an immune response.
- Bacterial or viral component. Instead of containing a whole microbe, these vaccines contain only parts of the microbe that trigger an immune response.
Combination Vaccines
These are vaccines that combine more than one vaccine into a single injection (shot). Combination vaccines for diphtheria, tetanus, and pertussis (DTaP) and for measles, mumps, and rubella (MMR) have been available for many years.
New combinations that cover up to 5 vaccinations have been developed. They are safe and well-tolerated in infants as young as 2 months. For example, one such vaccine combines DTaP, hepatitis B, and the polio vaccines. It is as effective as the individual vaccines and is often used in order to minimize the number of injections (shots) given at one time.
Passive Immunity
Another form of protection against disease is called passive immunity. This approach uses immune globulin, which is a blood product containing antibodies. Immune globulin may be used for people who cannot be vaccinated, when immediate protection is required, or to prevent severe complications of the disease. In some cases, passive immunization interferes with active vaccinations, particularly live-virus vaccines. Therefore, these two immunization types are usually not administered within several weeks of each other. One exception to this rule is in the case of high risk exposure to rabies.
Each year the US Centers for Disease Control and Prevention (CDC) issues updated immunization schedules for children and adults. A schedule is a list of recommended vaccines and timing of their doses. The current schedules can be found at: cdc.gov/vaccines/schedules/index.html. This article describes routine vaccines for children and adults.
Routine Childhood Vaccines
In the US, many vaccines are first given during infancy. Even most premature infants can be immunized on a routine schedule.
Experts recommend that all children through 18 years of age be routinely vaccinated against the following diseases:
- Measles
- Mumps
- Rubella (German measles)
- Diphtheria
- Tetanus
- Pertussis (whooping cough)
- Poliomyelitis (polio)
- Varicella (chickenpox)
- Hepatitis B
- Hepatitis A
- Haemophilus influenzae type b (Hib, a cause of meningitis)
- Human papillomavirus (HPV)
- Influenza (flu)
- Pneumococcal disease
- Meningococcal disease
- Rotavirus
Common Adult Vaccines
Vaccines against the following diseases are recommended for adults:
- Influenza (flu)
- Pneumococcal pneumonia
- Hepatitis A
- Hepatitis B
- Meningococcal meningitis
- Tetanus, diphtheria, and pertussis
- Measles, mumps, rubella
- Herpes zoster (shingles) vaccine
- Human papillomavirus (HPV)
- Varicella (chickenpox)
Inactivated-virus and toxoid vaccines are generally safe in pregnant women. Tdap is safe and recommended for all pregnant women in their third trimester to boost immunity across the placenta to the baby after birth. Other vaccines should be delayed until the second or third trimester. Because of possible risk to the fetus, live-virus vaccines should not be given to pregnant women or women likely to become pregnant within 28 days. The exception is women who need immediate protection against life-threatening diseases, such as yellow fever, which can only be prevented with live-virus vaccines. The live-virus MMR combination, which vaccinates against measles, mumps, and rubella, is not given to pregnant women because of risk of the live-rubella vaccine to the fetus. The injectable inactivated influenza vaccine (preservative-free subtype) is safe to administer to pregnant mothers during the fall and winter months prior to start and during the influenza season.
Vaccines are not completely effective for people whose immune systems are compromised by disease or medications. Immune globulin is often given if there is a significant risk of infection. Live-virus vaccines are not usually given to people who have weakened immune systems due to illness or medication.
People who should not receive live-virus vaccines include:
- Some people who have immune deficiency diseases
- Advanced HIV/AIDS (typically CD4 counts <200 cells/microliter)
- Patients with active leukemia or lymphoma.
- Patients who are receiving treatments that suppress the immune system, such as corticosteroids, alkylating drugs, antimetabolites, or radiation. (Exceptions are discussed below under individual vaccines.) Corticosteroids taken for less than 2 weeks should not affect live-virus vaccination. People who need vaccines and take long-term or high-dose topical steroids should check with their health care providers.
Some people worry that vaccines are not safe and may be harmful, such as for children. They may ask their provider to wait or even choose not to have the vaccine. But the benefits of vaccines far outweigh their risks. For example:
- After immunizations were introduced on a wide scale, infections such as tetanus, diphtheria, mumps, measles, pertussis (whooping cough), and polio became rare. All of these illnesses can cause lifetime disabilities or even death.
- Vaccines have also decreased certain types of meningitis, pneumonia, and ear infections in children.
- Pregnant women may contract infections that can be very dangerous to their fetus or newborn. Vaccines reduce this risk.
- High vaccination rates in a population confer what is called "herd immunity". The greater the proportion of people in a community getting vaccinated, the better protected is everyone in the community. Herd immunity reduces or stops disease transmission and thus reduces the risk of disease in those individuals who cannot receive the vaccine (such as pregnant women or people with a compromised immune system).
Autism
Scientific studies have shown that vaccines and their components do not cause autism. Based on this evidence, the American Academy of Pediatrics, the Centers for Disease Control and Prevention, and the Institute of Medicine all conclude that the benefits of vaccines outweigh their risks.
- Thimerosal is a preservative that was used in many vaccines since the 1930s. Preservatives are necessary to prevent vaccine contamination with live organism in vials that are used for vaccinating multiple people. Now, all vaccines recommended for children, except one type of influenza (flu) vaccine, contain no thimerosal. Multidose inactivated influenza vaccine has only a trace amount of thimerosal. (A trace amount means that a dose of vaccine contains less than 1 part per million.) The single-dose flu vaccine does not have any thimerosal.
- Autism is also not linked to the number of vaccines that children receive at a young age. From birth onward, children are exposed to substances called antigens that trigger an immune response. The number of antigens in vaccines is tiny as compared to the many antigens children are exposed to every day.
- The single 1998 small sample, uncontrolled study claiming that autism is linked to MMR vaccination has been thoroughly debunked by several large, controlled epidemiological studies. The lead author of the 1998 study was found guilty of ethical violations and of deliberately falsifying the study data. Although that article was retracted, this false claim persists and is still being spread on the internet. To find out the facts, visit the Centers for Disease Control and Prevention page on this subject.
Getting the Actual Infection From a Vaccine
Unless a person's immune system is weakened, it is highly unlikely that a vaccine will cause an infection. Some vaccines, such as the measles, mumps, rubella (MMR), the chickenpox, the rotavirus, and the nasal spray flu vaccines contain live but weakened viruses. These vaccines should not be given to people who have weakened immune systems.
Adverse Events
Like many medications, there is always a chance a vaccine can cause adverse events. An adverse event is a health problem caused by a medical treatment. An adverse event is also called a side effect.
No vaccine is 100% safe. Allergic reactions and other side effects may sometimes develop. In the US, the government and other agencies monitor side effects from vaccines:
- VAERS (Vaccine Adverse Event Reporting System) is a government service that registers all adverse events reported after vaccination, including those not related to the vaccine.
- VSD (Vaccine Safety Datalink) is a linked database that analyzes the records of more than 5 million patients each year.
- The CDC Clinical Immunization Safety Assessment Centers help providers evaluate and manage people who may have had a side effect from a vaccine.
- The Center for Biologics Evaluation and Research (CBER) is the FDA center responsible for ensuring the safety, effectiveness, and availability of biological products.
Studies using these systems are ongoing and none to date have confirmed reports of any significant link between most vaccines and severe side effects that would outweigh their important benefits.
Infants often accept the first shot (injection) easily, since they are not expecting any pain or discomfort. It gets more difficult, though, with each additional shot. Providing reassurance can help children of all ages tolerate immunizations. Here are some tips:
- Do not lie and tell an older child that a shot will be painless. Some providers suggest telling your child that it stings a little, and to count to 5 while the vaccine is being administered. A cooling spray of water can help numb the skin before the shot.
- Have your child take a deep breath right before the shot and blow out hard while it is being given.
- For children under 12 months, giving a sweet fluid before the shot may help ease the pain. Sugar has been found to relieve pain in infants.
- Ask the provider if you can give children's acetaminophen (Tylenol) or ibuprofen (Motrin, Advil) after a vaccination if your child has pain or fever. Do not give aspirin or medicine that has aspirin in it because aspirin can cause severe health problems in children.
If a person is ill with something more serious than a cold or develops a fever (if temperature is higher than 101°F [38.3 °C]), the vaccination may need to be scheduled for another day. Call your provider or immunization clinic for more information about this.
Severe Reactions to Vaccinations
Although severe reactions are very rare, you should know how to respond.
Call the provider right away if a child has any of the following symptoms.
- Extremely high fever. It is common for children to develop a low-grade fever in 1 to 2 days following vaccination. A rectal temperature of 105°F (40.5°C) or higher should prompt medical evaluation. (Temperatures taken under the arm or by mouth often register lower than actual temperatures.)
- Inconsolable crying. The child has been crying for over 3 hours without stopping or has a cry that is not normal, such as a high-pitched sound.
- Convulsions. The child's body starts shaking, twitching, or jerking. This reaction is usually due to a high fever. Lie the child down with the head to one side. Protect the head from hitting anything hard. Be sure the child can breathe freely. A simple febrile seizure usually stops by itself within a few seconds to 10 minutes.
Call 911 right away if a child has any of the following symptoms:
- Shock. The child collapses, turns pale, and becomes unresponsive.
- Severe allergic reaction (anaphylaxis). Swelling in the mouth and throat, wheezing and difficulty breathing, dizziness. The child collapses or is pale and limp.
- Seizure. The child is shaking uncontrollably.
Call the provider if the following symptoms persist for more than 24 hours:
- The injection site is still red and tender.
- Fever does not go down.
- The child is still fussy.
Adults who have any of the above side effects after vaccination should also seek medical attention.
After a severe reaction to a vaccine, such as an allergic reaction or convulsion, the person should not get any additional doses (shots) of the same vaccine. Call your provider or immunization clinic for more information about this.
Diphtheria, Tetanus, and Pertussis
Diphtheria
Diphtheria is caused by the toxin released by the bacterium Corynebacterium diphtheriae, which commonly infects the nose and throat. The throat infection leads to a gray to black, tough fiber-like membrane that can block the airway, making it hard to breathe, which can be life-threatening. In the early 1900s, diphtheria infected 200,000 people every year in the United States, killing up to 10% of infected people. Since a vaccine became available in the 1920s, there have been almost no cases of diphtheria in the United States.
Tetanus
Tetanus is a disease that causes severe muscular contractions and convulsions. It is caused by a powerful toxin secreted by the bacterium Clostridium tetani. People become infected by this dangerous bacterium through skin wounds. It is fatal in 15% to 40% of cases. Since widespread vaccination began in the late 1940s, new cases of tetanus have dropped by more than 95%.
Pertussis (whooping cough)
Pertussis was a very common childhood illness throughout the first half of the 20th century. It is caused by the bacterium Bordetella pertussis, which spreads easily from person to person. Pertussis causes uncontrollable, violent coughing attacks that can last for several weeks or months. In infants, it can cause permanent health problems and even death. Because of the vaccine, cases of whooping cough in the US reached an all-time low of 1,010 in 1976. The incidence has risen in recent years, with over 40,000 cases reported in 2012, and over 15,000 cases reported in 2018. The increase has occurred because immunity from the vaccine wears off by adolescence. Therefore, more cases develop in adolescents and adults as well as in infants younger than 2 months, who cannot receive the vaccine. For this reason, a booster dose has been added to the routine adult vaccination schedule.
DTaP
DTaP is the shortened name of the routine vaccine recommended for children through 6 years of age. DTaP is a combined vaccine made from inactivated (killed) bacteria or components that cause diphtheria, tetanus, and pertussis.
Children should get 5 doses (shots) of the vaccine. One dose should be received at the following ages:
- 2 months
- 4 months
- 6 months
- 15 to 18 months
- 4 to 6 years
DTaP can be given as an injection (shot) by itself, or it can be combined with other vaccines:
- DTaP-Hib-IPV (DTaP, inactivated poliovirus, and Haemophilus influenzae type b vaccine)
- DTaP-HepB-IPV (DTaP, hepatitis B, and inactivated poliovirus vaccine)
- DTaP-IPV (DTaP and inactivated poliovirus vaccine)
The advantage of a combined vaccine is that there are fewer shots. A provider can tell you if a combination vaccine is right for your child.
Tdap and Td
Because immunity to diphtheria, tetanus, and pertussis wears off, booster vaccinations are needed. Tdap and Td are used as booster vaccines:
- DTaP vaccine starts to wear off after about 5 years. Tdap booster is then given.
- Protection against diphtheria and tetanus lasts about 10 years. At that point, the Td booster is given. The Td vaccine contains the standard dose against tetanus and a less potent one against diphtheria. It does not contain the pertussis component.
People who should receive the Tdap or Td vaccines include:
- Children, 7 through 10 years old, who did not get any or all 5 DTaP vaccine shots (doses) between ages 2 and 6 years, need one shot of Tdap. If additional shots are needed, Td vaccine is given.
- Children, 11 through 12 years old need one of Tdap as a booster if they received all 5 DTaP shots when younger. The Td booster is then given every 10 years.
- All adults, 19 years and older, need a booster shot of Td every 10 years.
- All adults, 65 years and older, who have never had Tdap should get one dose as their next booster.
- Pregnant women need one shot of Tdap during 27 to 36 weeks of each pregnancy. This vaccination is to protect the mother from getting the infections, such as pertussis, and spreading the germs to her newborn. (Babies younger than 2 months cannot get the DTaP vaccine because it is not safe for them.)
- The father or partner of the mother or caregiver should also receive the TDaP vaccine prior to birth, to help prevent a potential exposure to pertussis of newborns, who are not able to receive the DTaP vaccine until 8 weeks of age.
- Children and adults who have a severe cut or burn may need Td or Tdap to protect against tetanus infection.
- People who received a dose (shot) of any vaccine that has tetanus, diphtheria, or pertussis in it and developed an allergic reaction to the vaccine.
- People who went into a coma or developed seizures within 1 week of getting a dose of DTaP or the older vaccine called DTP should not receive Tdap. These people may be able to receive Td.
- People who have nervous system problems, such as epilepsy, should ask their providers about receiving any diphtheria, tetanus, pertussis vaccines.
- People who have Guillain-Barré syndrome should ask their providers about receiving any diphtheria, tetanus, pertussis vaccines.
- People who are ill with something more severe than a cold or who have a fever should reschedule their vaccinations for after they have recovered.
Common side effects include:
- Pain and swelling at the injection site
- Mild fever
- Irritability in children
Severe side effects include allergic reaction.
Measles, Mumps, and Rubella
Measles
Measles (rubeola) is one of the most contagious human infections. It is caused by a virus. Measles used to be a very common childhood disease. Most cases resolve without serious complications. Symptoms include fever, cough, runny nose, and rash that covers the whole body. In severe cases, measles can cause pneumonia, encephalitis (inflammation in the brain), or death. Risk of these severe complications is highest in the very young and very old. In pregnant women, measles increases the rates of miscarriage, low birth weight, and birth defects.
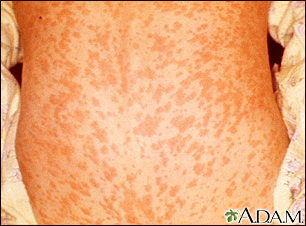
About 3 to 4 million people used to get measles yearly in the United States before vaccination started, and there were thousands of cases of measles-caused encephalitis or death each year. Vaccination programs have drastically reduced the incidence of measles in the US. But measles outbreaks still occur, usually starting with international travelers and being spread among groups of people who decline immunizations or in areas where immunization levels have decreased. In 2019, 1282 cases of measles were reported in the US, the largest number of cases in a year since 1994, over 70% of which occurred in unvaccinated individuals. Vaccination rates have stagnated worldwide in recent years. Outbreaks in 2018 led to over 140,000 measles deaths, mostly among children under 5 years of age.
Mumps
Mumps is caused by a virus. The infection may cause no symptoms. If symptoms occur, they include fever, headache, sore throat, and parotitis, or painful swelling of one or both parotid salivary glands (located between the ear and jaw). In some cases, mumps affects the lining of the brain and spinal cord, although this is usually not permanently harmful. Swelling of the testicles occurs in some males who have reached puberty, although sterility is rare. Deafness in one ear occurs very rarely.
Rubella (German Measles)
Rubella is caused by a virus. The infection leads to a mild illness that includes a rash, enlarged lymph nodes, and sometimes a fever. If a pregnant woman is infected during her first trimester, her baby has an 80% chance of developing birth defects, including heart abnormalities, cataracts, deafness, and learning disabilities.
Vaccination programs have dramatically reduced the number of mumps and rubella cases in the United States. Some adults are still susceptible, particularly immigrants who were not vaccinated in their birth countries.
MMR
The measles, mumps, rubella vaccine is called MMR for short. It is a routine vaccine recommended for children through 6 years old. MMR is a combined vaccine made from live but very weak viruses that cause the three diseases.
Children should get 2 doses (shots) of the vaccine. One dose should be received at the following ages:
- 12 to 15 months old. To make sure the child is properly protected, the vaccine must not be given too early.
- 4 to 6 years old before starting school. But the shot can be given at any time after that.
In addition, prior to traveling outside the US, infants younger than 12 months old should get an early dose of the MMR vaccine at age 6 to 11 months old. These children should subsequently follow the recommended schedule and get another dose at 12 to 15 months old and a final dose at 4 to 6 years old.
Adults born during or before 1956 in the United States are considered immune because they likely had the actual diseases during childhood.
Adults -- 18 years old, or who were born after 1956 -- should get at least one shot of the MMR vaccine if:
- They have never received an MMR shot
- They are not sure whether or when they received an MMR shot
- They have never had any of the three diseases
People who received inactivated types of measles or mumps vaccine in the 1960s or 1970s should be revaccinated with two doses of the live MMR vaccine.
The American Academy of Pediatrics recommends the MMR vaccine for HIV-infected children, teenagers, and young adults, except for those who are severely immunocompromised. The vaccine appears to be safe in HIV-infected children. Measles infection is very dangerous in this population.
Women who can get pregnant and who have not had the MMR vaccine in the past should have a blood test to see if they are immune. Being immune means, they have had the three diseases or the vaccine in the past and are now protected. If they are not immune, they should receive the MMR vaccine. Women should not get this vaccine if they are pregnant or planning to become pregnant within 4 weeks. The vaccine may harm the baby.
MMRV
The MMRV combines the MMR vaccine with the chickenpox (varicella) vaccine into a single vaccine. One dose (shot) of MMRV is given to children at the following ages:
- 12 to 15 months
- 4 to 6 years
The advantage of the MMRV vaccine is that the child gets one less shot. The disadvantage is a higher chance of developing a fever of 102°F (38.8°C) or higher and seizures than with getting the MMR and chickenpox vaccines separately. Talk with the provider about whether the MMRV vaccine is right for your child.
The MMRV vaccine is not given to people 13 years and older.
- People who received a dose of MMR, MMRV, or the chickenpox vaccine (VAR) and developed an allergic reaction to it.
- People who are severely allergic to the antibiotic neomycin. Both the MMR and MMRV vaccines contain tiny amounts of neomycin.
- Pregnant women should not receive MMR.
- People 13 years and older cannot receive MMRV.
- Parents of children who have immune system problems, such as HIV/AIDS, or weakened immune systems should check with their providers before their children receive MMR or MMRV.
- People who are ill with something more severe than a cold or have a fever should reschedule their vaccination for after they have recovered.
Common side effects include:
- Fever
- Rash and itching
- Mild swelling of the salivary glands
- Joint pain
Chickenpox
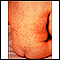
Chickenpox is caused by the varicella-zoster virus. Chickenpox is one of the most contagious childhood diseases. Nearly every unvaccinated child becomes infected with it. Symptoms include itchy, fluid-filled blisters all over the body that burst and form crusts.
The infection rarely causes complications in healthy children. In severe cases, though, children need to be hospitalized for treatment.
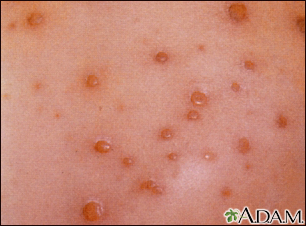
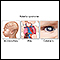
This is a close-up picture of chickenpox. Early chickenpox lesions consist of small red papules that quickly fill with a yellowish or straw-colored fluid to form small blisters (vesicles), as seen in this photograph. Later, these vesicles will rupture, forming shallow erosions that crust over and then ultimately heal.
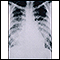
This chest x-ray shows cloudiness throughout the lungs, caused by acute pneumonia following chickenpox. Pneumonia, as a complication of chickenpox, rarely occurs in children, but is more frequent in adults.
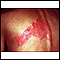
Chickenpox can be serious in adults and in people with compromised immune systems. It can lead to severe pneumonia, encephalitis (infection of the brain), or even death. In people who had chickenpox as children, the virus stays quiet (dormant) in the nerves of the body for the rest of a person's life. When the virus becomes active, it causes areas of painful blisters. This condition is called shingles.
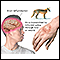
This image depicts a classical pattern for shingles. The infection follows a nerve root from the spine, along a rib, to the front of the chest. The area innervated by the nerve is called a "dermatome".
VAR
The chickenpox (varicella) vaccine is called VAR for short. It is made from weakened chickenpox virus. After getting the vaccine, a person is protected from getting chickenpox. Or if a person does get chickenpox, the infection is usually mild. The vaccine can also prevent chickenpox or reduce its severity if it is used within 3 days, and possibly up to 5 days, after exposure to the infection.
VariZIG, a varicella-zoster immune globulin was approved in 2012 for high risk individuals exposed to the virus. High risk candidates include:
- Certain immunocompromised individuals
- Pregnant women without evidence of immunity
- Certain newborns and premature infants
It is the only one of its kind available in the United States. Ideally, it is administered within 4 days of exposure, but may be given within 10 days.
Recommendations for Young Children
VAR is a routine vaccine recommended for children through 6 years old. Children should get 2 doses (shots) of the vaccine. One dose should be received at the following ages:
- 12 to 15 months old.
- 4 to 6 years old before starting school. This second dose can be received before 4 years old, as long as it is 3 months after the first dose. It is also acceptable to get the second dose as soon as 4 weeks after the first dose.
Recommendations for People 13 Years and Older:
- People who have not received the vaccine and have not had chickenpox should get 2 doses (shots). The second dose should come at least 4 weeks after the first dose.
- People who have had only 1 dose and have not had chickenpox should get a second dose. The second dose should come at least 4 weeks after the first dose.
Healthy adults without a known history of chickenpox, and who have had a blood test that does not show immunity, should receive 2 doses of the vaccine, including the following groups:
- Older people without a history of chickenpox and who are at high risk of exposure or transmission (such as hospital or daycare workers and parents of young children)
- People who live or work in environments in which viral transmission is likely
- Non-pregnant women of childbearing age
- Adolescents and adults living in households with children
- International travelers
MMRV
MMRV combines the chickenpox vaccine with the measles, mumps, rubella (MMR) vaccine into a single vaccine. One dose (shot) of MMRV is given to children at the following ages:
- 12 to 15 months
- 4 to 6 years
The advantage of the MMRV vaccine is that the child gets one less shot. The disadvantage is a higher chance of developing a fever of 102°F (38.8°C) or higher and seizures than with getting the MMR and chickenpox vaccines separately. Talk with your provider about whether the MMRV vaccine is right for your child.
The MMRV vaccine is not given to people 13 years and older.
- Pregnant women, including the 3 months prior to pregnancy. (This applies to VAR. MMRV is not given to people 13 years and older.)
- Certain HIV infected individuals (CD4 counts < 200 cells/microliter).
- People whose immune systems are compromised by disease or medication (such as after organ transplant, receiving chemotherapy, or on long term immunosuppressants such as steroids).
- People who are ill with something more severe than a cold or have a fever should reschedule their vaccination for after they have recovered.
People who cannot be vaccinated, but are exposed to chickenpox, may receive immune globulin against the varicella virus. When received within 4 days of exposure, it may help reduce the symptoms of chickenpox.
Common side effects at the injection site include:
- Pain
- Swelling
- Redness
Sometimes a mild rash develops within a month of vaccination, which in very rare cases, may spread chickenpox to others. People who have recently been vaccinated should avoid close contact with anyone who might be susceptible to severe complications from chickenpox until the risk has passed.
Severe side effects include an allergic reaction.
For more information, see the in-depth report on shingles and chickenpox (Varicella-zoster virus).
Shingles
Shingles is an infection caused by the varicella-zoster virus. This is the same virus that causes chickenpox. After a person gets chickenpox, the virus stays inactive (dormant) in the body. Years later, if the virus becomes active, it can cause shingles.
Shingles is most common in adults over age 50. It causes a painful, red, and sometimes blistery rash to form on the body or face. Other symptoms include:
- Headache
- Fever
- Chills
In rare cases, complications, such as pneumonia, blindness, and brain inflammation (encephalitis), can occur. In some people, after the rash goes away, the intense pain persists. This pain is called post-herpetic neuralgia (PHN).
The shingles (zoster) vaccine is recommended for healthy adults age 50 years or older, regardless of whether they have had shingles.
There are two shingles vaccines available in the United States:
- Recombinant zoster vaccine (RZV, Shingrix) is recommended as the preferred shingles vaccine. Shingrix is given to healthy adults 50 years and older in two doses separated by 2 to 6 months. Shingrix is more than 90% effective at preventing shingles and PHN.
- Zoster vaccine live (ZVL, Zostavax) may be used in healthy adults 60 years and older. Zostavax is given in a single dose. It may be preferred in cases of allergy to Shingrix or when a person requests immediate vaccination and Shingrix is not available.
- Anyone who has a weakened immune system due to HIV/AIDS or cancer of the lymph, bone, or blood, or due to treatments such as radiation or long-term corticosteroids.
- Women who are pregnant, or anyone who is in close contact with a pregnant woman who has not had chickenpox.
- People who are ill with something more severe than a cold or have a fever should reschedule their vaccination for after they have recovered.
- People who currently have shingles.
Common side effects at the injection site include:
- Mild redness
- Soreness
- Swelling
- Itching
Some people experience headache or several days of shingles-like neuralgia symptoms.
Hepatitis A
Hepatitis A is a contagious disease caused by a virus that causes liver inflammation. The virus is excreted in feces and spreads when a person eats food or drinks water contaminated by the virus. Eating raw shellfish taken from sewage-contaminated water is a common means of contracting hepatitis A. It can also be acquired by close contact with people infected with the virus, such as in daycare centers.
HepA
The hepatitis A vaccine is called HepA for short. HepA is made from the inactivated virus.
Children should get 2 shots (doses) of HepA between 12 and 23 months old. The 2 shots should be separated by 6 to 18 months. Children 2 through 18 years old should get the 2 doses of HepA if they live in an area where many people have hepatitis A infection.
The vaccine can also be effective when given within 2 weeks of a known exposure.
Adults, 19 years and older, should get the 2 doses of HepA if they:
- Work or travel in areas where hepatitis A is common. These areas include Africa, Asia (except Japan), the Mediterranean, Eastern Europe, the Middle East, Central and South America, Mexico, and parts of the Caribbean.
- Use illegal injectable or non-injectable drugs.
- Work with the hepatitis A virus in a laboratory or with research animals that are infected with the virus.
- Have long-term (chronic) liver disease.
- Adopt children from a country where many people have hepatitis A.
- Are men who have sex with other men.
- Are pregnant and at high risk of infection or of severe outcome if infected during pregnancy.
Hepatitis A Inactivated and Hepatitis B (Recombinant)
This vaccine combines the hepatitis A and hepatitis B vaccines into a single vaccine. The brand name of this vaccine is TWINRIX.
- It can be given to children starting at 1 year old.
- It can also be given to teens and adults.
- Depending on age and need, it is given in 3 or 4 shots (doses), with each shot separated by a certain period of time.
- People who have had hepatitis A infection do not need the vaccine because they are immune for life.
- People who received a dose of the vaccine and developed an allergic reaction to it.
- Pregnant women should ask their provider if the vaccine is safe for them.
- People who are allergic to neomycin should not receive TWINRIX, which contains a tiny amount of neomycin.
- People who are ill with something more severe than a cold or have a fever should reschedule their vaccination for after they have recovered.
The most common side effects are:
- Soreness at the injection site
- Headache
- Fatigue
- Loss of appetite
The most serious side effect is an allergic reaction.
Hepatitis B
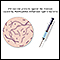
Hepatitis B is a serious disease that causes life-threatening liver damage. Worldwide about 350 million people are infected with the hepatitis B virus. Each year 780,000 people die worldwide, mostly due to cirrhosis and liver cancer caused by long-term (chronic) hepatitis B infection. In the US, about 1.2 million people have chronic hepatitis B and up to 80,000 become newly infected each year.
Hepatitis B is also known as serum hepatitis. It spreads through blood and sexual contact. The infection is seen with increased frequency among intravenous drug users who share needles and among the homosexual population. The photo above is an electron microscopy image of hepatitis B virus particles. (Courtesy of the CDC.)
Hepatitis B spreads through blood and sexual contact. People at high risk of the disease include:
- Injection drug users
- People who have sex with someone who has hepatitis B
- People who live with someone who has hepatitis B or has a positive hepatitis B surface antigen test
- Health care workers at risk for exposure to blood or blood-contaminated body fluids
- People who are treated in facilities where a high proportion of adults have a high risk for hepatitis B
Pregnant women with hepatitis B can transmit the virus to their babies. Even if they are not infected at birth, unvaccinated children of infected mothers can get hepatitis B.
Hepatitis B infections have been greatly reduced since routine childhood immunization began in the early 1990s. But there are still children who are not immunized, and the disease persists. Routine vaccination against this disease during childhood is very important.
HepB
The hepatitis B vaccine is called HepB for short. HepB is made from the inactivated virus.
Recommendations for Childhood
HepB is a routine vaccine recommended for infants through 18 months old. Three doses (shots) of HepB should be received at the following ages:
- Soon after birth and before hospital discharge
- 1 to 2 months old
- 6 to 18 months old
Infants of mothers infected with hepatitis B should be treated with immune globulin plus HepB within 12 hours of birth. The second dose of HepB should be received at 1 to 2 months old and the third dose at 6 months old.
Infants born to infected mothers should be tested for antibody status at 9 to 18 months to see if they are long-term (chronic) virus carriers or need to be revaccinated.
When it is not known if a mother is infected, the infant should receive the vaccine within 12 hours of birth. The mother's blood should then be tested right away. If she is infected, the infant should receive immune globulin within 1 week of birth.
Hepatitis B Vaccine for Adults
HepB is received in 3 doses (shots) over 6 months. It is routinely recommended for all unvaccinated children aged less than 19 years. In addition, the following adult groups are at high risk and should be vaccinated:
- Health care and public safety workers who may be exposed to blood products
- Sex partners of people with hepatitis B (positive hepatitis B surface antigen test)
- People in the same household as someone infected with hepatitis B
- Travelers to countries with a high incidence of hepatitis B infection
- Sexually active individuals with multiple partners
- People with any sexually transmitted diseases
- People with diabetes, under 60 years of age, or older diabetic people who use glucose monitoring devices or have other risk factors
Other people at risk who would benefit from vaccination include:
- Patients and workers in mental institutions
- Morticians
- People undergoing kidney dialysis
- People who use injected drugs
- Pregnant women at risk of the virus (there is no evidence that the vaccine is dangerous to the fetus)
- People with HIV or with hepatitis C virus infection
Hepatitis A Inactivated and Hepatitis B (Recombinant)
This vaccine combines the hepatitis B and hepatitis A vaccines into a single vaccine. The brand name of this vaccine is TWINRIX.
- It can be given to children starting at 1 year old.
- It can also be given to teens and adults.
- Depending on need, it is given in 2, 3, or 4 shots (doses), with each shot separated by a certain period of time.
Your provider can tell you if this vaccine is right for your child or you.
DTaP-HepB-IPV
This vaccine combines HepB with two other vaccines: diphtheria, tetanus, pertussis (DTaP), and polio (IPV). It is for children 6 weeks through 6 years of age. Three doses (shots) are recommended at 2, 4, and 6 months of age. The advantage of this vaccine is that it reduces the number of injections a child has to receive. Talk with the provider about whether the DTaP-HepB-IPV vaccine is right for your child.
- People who are allergic to yeast.
- People who received a dose of the vaccine and developed an allergic reaction to the vaccine.
- People who are allergic to the antibiotics neomycin and polymyxin B should not receive TWINRIX or DTaP-HepB-IPV. The vaccines contain tiny amounts of these antibiotics.
- Children who have progressive neurologic problems, such as uncontrolled epilepsy, should not receive the DTaP-HepB-IPV until the problem is stabilized.
- People who are ill with something more severe than a cold or have a fever should reschedule their vaccination for after they have recovered.
The most common side effects are fever or soreness at the injection site. The most serious side effect is an allergic reaction.
For more information, see the in-depth report on hepatitis.
Pneumococcal Disease
Pneumococcal disease is caused by the pneumococcus bacterium, Streptococcus pneumoniae (S pneumoniae). Different types of pneumococcal disease include respiratory infections, blood infections, ear infections, and meningitis. The most common type of severe pneumococcal disease is pneumonia.
People at highest risk of pneumococcal disease are:
- Children younger than 5 years old
- Older adults
- Those who have long-term (chronic) health problems such as diabetes mellitus, heart disease, or lung disease
- People whose immune systems are weakened
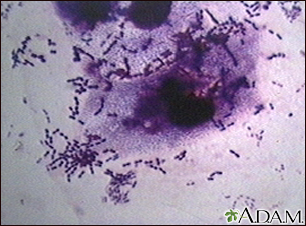
This photo shows the organism pneumococci. These bacteria are usually paired (diplococci) or appear in chains. Pneumococci are typically associated with pneumonia, but may cause infection in other organs, such as the brain (pneumococcal meningitis) and bloodstream (pneumococcal septicemia). (Courtesy of the CDC.)
PCV13
The pneumococcal conjugate vaccine protects against 13 of the most severe types (strains) of the over 90 strains of S pneumoniae. For this reason, this vaccine is called PCV13 for short. These 13 strains affect mainly children and about half of adults who get pneumococcal disease. PCV13 is made with components of the 13 S pneumoniae strains. The CDC recommends PCV13 vaccination for all children younger than 2 years, all adults older than 65 years, and people ages 2 to 64 years with certain medical conditions.
PCV13 is one of the recommended childhood immunizations. Children should get 4 doses (shots) of PCV13. One dose should be received at:
- 2 months old
- 4 months old
- 6 months old
- 12 to 15 months old
One dose of PCV13 and one dose of PPSV23 may be given to older children or adults with weakened immune systems due to health conditions such as HIV or kidney and spleen problems. People with cochlear implants may also need the vaccines.
PPSV23
The pneumococcal polysaccharide vaccine is called PPSV23 for short. It protects against the 23 strains of S pneumoniae that cause the most severe infections. PPSV23 is made from components of 23 S pneumoniae bacteria strains without extra protein. PPSV23 is not fully effective in people who are very old or have long-term (chronic) health problems.
PPSV23 is recommended for the following older children and adults:
- Anyone older than 2 years of age with heart disease; lung disease (including asthma); kidney disease; people on dialysis; alcoholics; those who have leaks of cerebrospinal fluid; or people with diabetes, cirrhosis, or cochlear implants.
- All people over 65 years old. People over 65 who received a dose of PPSV23 before they were 65 should receive a second dose after they turn 65.
- Adults aged 19 through 64 years who have asthma or smoke should receive a single dose of PPSV23.
- Those with sickle cell disease.
- Those with a dysfunctional spleen and those who have had their spleens removed. A second dose should be received 5 years or more after the first dose.
- People with conditions that weaken the immune system, such as leukemia or other cancers, HIV infection, or organ transplantation.
- People who receive chronic (long-term) immunosuppressive medications, including steroids.
- Individuals with immune deficiencies or those undergoing treatments that suppress the immune system. A second dose should be received 5 years or more after the first dose.
- People with autoimmune diseases, such as rheumatoid arthritis and lupus, although protection may not be as strong for these people.
- Older people who have had transplant operations or those with kidney disease may require a revaccination after 5 years.
- People living in long-term care facilities.
- Alaska Natives or Native Americans over age 65 years who live in areas where pneumococcal disease is common. In certain communities, people 50 through 64 years old or younger may need the vaccine.
Your provider can tell you about the timing of the PCV13 and PPSV23 vaccines.
- People who received a dose of the vaccine and developed an allergic reaction to it.
- People who had an allergic reaction to an older pneumococcal vaccine called PCV7, or from any diphtheria vaccine (DTaP, Tdap, or Td) should not receive PCV13.
- Pregnant women should ask their providers if the vaccine is safe for them.
- People who are ill with something more severe than a cold or have a fever should reschedule their vaccinations for after they have recovered.
Common side effects include:
- Mild pain and redness at the injection site
- Joint aches
- Fever
Children are more likely to have fever within 48 hours if they receive other vaccines at the same time, and also after the second dose.
Poliomyelitis
Poliomyelitis is commonly known as polio. The disease is caused by a virus called poliovirus and can lead to paralyzing nerve damage, which can be fatal or lead to lifetime disability. Polio was a major killer of children in the early 20th century. Vaccination programs have eliminated the disease in most parts of the world. But cases still occur in certain parts of Asia and Africa. Vaccination is still recommended because there is still a risk of acquiring polio through international travel.
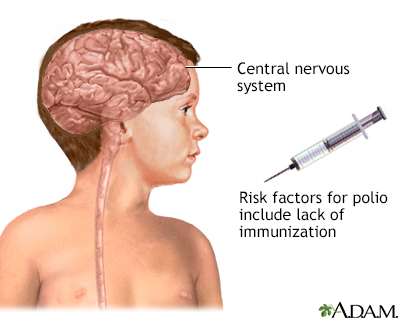
Poliomyelitis is a communicable disease caused by viral infection and occurs through direct contact with infected secretions. Polio is found worldwide, but immunization has reduced the incidence. Clinical polio affects the central nervous system (brain and spinal cord). Disability is more common than death.
IPV
The polio vaccine is made from inactivated poliovirus. For this reason, the vaccine is called IPV for short. IPV is given as an injection. The oral polio vaccine is no longer used in the United States.
Polio vaccine is one of the recommended childhood immunizations. Children should get 1 dose (shot) of IPV at:
- 2 months old
- 4 months old
- 6 to 18 months old
- 4 to 6 years old
Children who have received 3 doses of the IPV before age 4 should get the fourth dose before or at the time they start school. The fourth dose is not needed if the third dose is received after age 4.
Combination Vaccines
The polio vaccine can be given as a shot by itself. Or it can be combined with another vaccine:
- DTaP-Hib-IPV (DTaP, Haemophilus influenzae type b, and inactivated poliovirus vaccine)
- DTaP-HepB-IPV (DTaP, hepatitis B, and inactivated poliovirus vaccine)
- DTaP-IPV (DTaP and inactivated poliovirus vaccine)
The advantage of a combined vaccine is that there are fewer shots. The provider can tell you if the combined vaccine is right for your child.
- The polio vaccine is not usually recommended for people over 18 years. Exceptions are unvaccinated health care workers, laboratory technicians, or others who may be exposed to the virus.
- People who received a dose of the vaccine and developed an allergy to it.
- People who are allergic to the antibiotics neomycin, streptomycin, or polymyxin B. The vaccine contains tiny amounts of these antibiotics.
- Children who have progressive neurologic problems, such as uncontrolled epilepsy, should not receive any of the combined vaccines until the problem is stabilized.
- People who are ill with something more severe than a cold or have a fever should reschedule their vaccinations for after they have recovered.
The most common side effects are soreness at the injection site or fever. Serious side effects may include an allergic reaction.
Influenza
Influenza is also known as the flu. It is caused by the influenza virus. The virus spreads easily from person to person. The flu causes a sore throat, fever and chills, headache, muscle ache, cough, and fatigue. Influenza is responsible for over 200,000 hospitalizations a year in the US and results in thousands of deaths.
Pneumonia is a major serious complication of the flu. It nearly always occurs in high-risk people such as the very young or very old and pregnant women. People who are hospitalized or whose immune systems are weakened are also at high risk of complications.
Influenza, also known as the flu, is caused by a virus.
There are 3 types of the influenza virus that can infect humans:
- Type A is the most widespread. It can affect both animals and humans. In humans, it can cause severe illness. Influenza A is the cause of all the worldwide outbreaks (pandemics) of the flu. In recent years, subtypes of influenza A that were only known to infect animals, such as the avian (bird) flu viruses, have begun to infect humans. The new subtype A virus, 2009 H1N1, which was discovered in 2009, causes severe illness.
- Type B infects only humans and is also responsible for seasonal epidemics. Young children are mostly affected by type B. Flu caused by this strain tends to be milder than that caused by influenza A.
- Type C also infects humans but is not thought to cause epidemics. Like type B, it mainly affects young children and the illness is mild.
Each influenza virus has different subtypes or strains. The seasonal flu vaccines work by protecting against those strains which experts think will be the most common for that season.
The flu vaccine is recommended for everyone 6 months and older.
However, it is most important for people at increased risk for complications from influenza infection, and for people who care for them. This includes:
- Children 6 through 59 months of age
- Adults 50 years of age and older
- Anyone with long-term (chronic) lung of cardiovascular phenol, liver, neurologic, hematologic, and diabetes disease
- People who are immunosuppressed
- Woman who are or will be pregnant during the influenza season
- Children or adolescents who are receiving long-term aspirin therapy
- Residents of nursing homes and other long-term care facilities
- American Indians and Alaska natives
- Morbidly obese individuals (BMI greater than 40)
- People who live with or care for people who are at high risk
For more information, see the in-depth report on colds and the flu.
The flu viruses are constantly changing their make-up. Scientists develop new vaccines each year to protect against the new flu strains that are expected to strike. The ability of the virus to change rapidly is why people need to receive a new vaccine each year.
People should get vaccinated each year before flu season starts. This is usually in September or October. One dose of the vaccine is needed. Children, 6 months through 8 years old, who are getting a flu vaccine for the first time need 2 doses.
The vaccine can be received as an injection (shot) or as a nasal spray. The injection can be delivered under the skin (intradermal) or into the muscle (intramuscular).
Injection
The flu shot contains killed (inactive) viruses. It is not possible to get the flu from this type of vaccine. Ahead of each flu season, the CDC makes the recommendation for the type of flu shot that will be recommended. Examples include:
- The regular flu shot is approved for people 6 months and older. This shot is injected into the upper arm muscle in adults and into the thigh muscle in babies and toddlers. The flu shot is designed to protect against 3 strains (trivalent flu vaccine) or 4 strains (quadrivalent flu vaccine) of influenza viruses.
- A high-dose version of the flu shot, called Fluzone High Dose, can be received by people 65 and older. As people age, their immune systems get weaker. The high-dose vaccine is designed to produce a stronger immune response to the flu virus. Evidence shows this dose is better at protecting older adults from the flu than the regular vaccine. Another flu shot designed for people 65 and older is the adjuvant vaccine, a standard trivalent vaccine containing additional components to increase the immune response.
- An intradermal flu shot, called Fluzone Intradermal, can be received by people 18 through 64 years old. The intradermal shot is injected under the skin. It uses a smaller and shorter needle. (The regular shot is injected into the muscle.) The intradermal shot also uses fewer antigens (inactive viruses). But it still protects against the flu like the regular shot.
- Flucelvax and Flublok are 2 new flu vaccines that are cell-based. This means the flu viruses in the vaccines are grown using cell culture technology instead of chicken eggs, which are used in the regular flu vaccine. Cell-based vaccines are made faster than traditional egg-based vaccines. Flucelvax can be received by people 18 years and older. Flublok can be received by people 18 through 49 years of age. Flublok is the only flu vaccine that can be given to people with egg or latex allergy. Currently, there is no safety information about the two vaccines for pregnant or nursing women. Your provider can tell you if either vaccine is right for you.
Nasal Spray
Sometimes the seasonal flu vaccine recommendation includes a nasal spray option. The nasal spray flu vaccine is made from live, weakened viruses. It is approved for healthy people ages 2 through 49 years, who are not pregnant. There is no data that suggests the live virus in the nasal spray is any more effective. Depending on the circulating strains, this form of the vaccine may not be recommended. Talk to your provider to find out if the nasal spray is advised.
People who are severely ill or have a fever should reschedule their flu vaccinations for after they have recovered.
Injection
People who should not get the regular vaccine include:
- Children younger than 6 months old.
- People who are severely allergic to eggs.
- People who have had a severe allergic reaction to the regular flu shot in the past.
- People who developed Guillain-Barre syndrome after getting the flu vaccine in the past.
- The intradermal vaccine cannot be received by people younger than 18 years or older than 64 years.
- The high-dose vaccine should not be received by people younger than 65 years old.
Flucelvax and Flublok
People who should not get Flucelvax or Flublok include:
- People who developed Guillain-Barre syndrome after getting any flu vaccine in the past.
- People who are allergic to any component in the vaccines.
- Flucelvax cannot be received by children younger than 18 years old, or who are allergic to rubber latex.
- Flublok cannot be received by people younger than 18 years or older than 64 years.
Nasal Spray
People who should not get the nasal spray include:
- Children 23 months old or younger, or adults 50 years and older.
- People who are severely allergic to eggs.
- People who have had a severe allergic reaction to the nasal vaccine in the past.
- Pregnant women.
- People who have asthma and children 4 years and younger who have had at least one episode of wheezing in the past year.
- People who have a nasal condition that makes it hard to breathe, such as a stuffy nose.
- People who have a weakened immune system.
- Children who take aspirin under a doctor's direction (most children should not take aspirin).
Possible side effects of the flu vaccines include:
- Allergic reaction to components in the vaccines, such as the egg-based vaccine.
- Soreness, swelling, or redness at the injection site.
- Runny nose, nasal congestion, sore throat, or fever with nasal spray vaccine.
- Flu-like symptoms. Some people actually experience flu-like symptoms, called oculorespiratory syndrome, which include conjunctivitis, coughing, wheezing, tightness in the chest, sore throat, or a combination. Such symptoms tend to occur 2 to 24 hours after the vaccination and generally last for up to 2 days. These symptoms are not the flu itself, but an immune response to the virus proteins in the vaccine.
- Guillain-Barre syndrome. This paralytic illness occurs in very rare cases.
Haemophilus influenzae Type B
Haemophilus influenzae (H influenzae) type b is a bacterium that commonly caused childhood bacterial meningitis, pneumonia, blood infection, and epiglottitis (throat swelling that can block breathing). Despite its name, this bacterium is entirely different from the viruses that cause influenza (the flu). Prior to the vaccine, about 600 children died of Hib infections every year in the US. Because of routine vaccination, serious Hib disease in children is now rare.
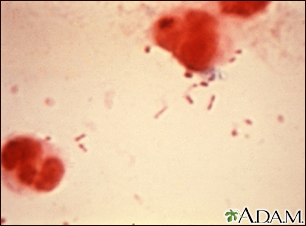
The photo above is a Gram stain of spinal fluid from a person with meningitis. The rod-like organisms seen in the fluid are H influenzae, one of the most common causes of childhood meningitis (prior to the widespread use of the H influenzae vaccine). The large red-colored objects are cells in the spinal fluid. A vaccine to prevent infection by H influenzae type b is available as one of the routine childhood immunizations (Hib), typically given at 2, 4, and 12 months.
Hib vaccine is highly effective in protecting against Hib disease. It is a routine vaccine recommended for children through 5 years old. Two vaccine brands are available. Depending on which vaccine is received, 3 or 4 doses (shots) are received.
Hib can be given as a shot by itself, or it can be combined with another vaccine:
- DTaP-Hib-IPV (DTaP, Haemophilus influenzae type b, and inactivated poliovirus vaccine)
- HepB-Hib (hepatitis B and Haemophilus influenzae type b vaccine)
Your provider can tell you whether the combined vaccine is right for your child.
Adults with certain health problems, such as sickle cell disease, or conditions affecting the spleen, should have the Hib vaccine if they have not already been vaccinated. Hematopoietic stem cell transplant recipients should also receive a 3-dose regimen 6 to 12 months after transplantation, regardless of vaccination history.
- Infants younger than 6 weeks old.
- People who received a dose of the vaccine and developed an allergic reaction from it.
- People who are ill with something more severe than a cold or have a fever should reschedule their vaccinations for after they have recovered.
- Hib vaccines are no longer recommended for people with HIV.
Side effects of the Hib vaccines include:
- Redness and pain at the injection site
- Moderate fever
- Weakness (rare)
- Nausea (rare)
- Dizziness (rare)
Human Papillomavirus (HPV)
HPV is actually a group of more than 150 viruses, about 40 of which are sexually transmitted. Some HPV strains can cause genital warts, cervical cancer, and cancers of the vulva, vagina, anus, and penis. HPV infection is very common. About 20 million people in the US have it. At least half of all sexually active men and women will eventually be exposed to the virus.
The HPV vaccine is made from inactivated human papillomaviruses. The vaccines protect against HPV strains 16 and 18, which account for 70% of cervical cancer cases in the United States.
HPV9
HPV9 is also known by its brand name Gardasil-9. The vaccine protects against 9 types (strains) of HPV.
It is recommended for both males and females. It is routinely given at 11 or 12 years of age, but it may be given in beginning at age 9 years through age 26 years.
Current Immunization Guidelines Recommend
Girls ages 11 and 12 years should receive the HPV vaccine series:
- The vaccine is given in 2 shots over a 12 month period if started before age 15 years. After age 15 years, 3 shots are required.
- The HPV vaccine can be given at the same time as other vaccines.
- Girls as young as age 9 years can receive the vaccine if their provider recommends it, such as children who have been abuse.
Girls and women ages 15 to 26 years:
- Those who have not received the HPV vaccine in the past should get a series of 3 shots.
- Those who have not completed the full vaccine series should catch up on missed shots.
- Pregnant women should not receive this vaccine. However, there have been no problems found in women who received the vaccine during pregnancy, before they knew they were pregnant.
Boys ages 11 to 12 years should receive the Gardasil-9 vaccine series:
- To reduce the chance of becoming infected with genital and anal warts. The vaccine also reduces the risk of cancer of the penis and anus.
- The vaccine is given in 2 shots over a 12 month period.
- Boys as young as age 9 years can receive the vaccine if their provider recommends it.
Boys and men ages 15 to 21 years:
- Those who have not received the vaccine in the past should get a series of 3 shots.
- Those who have not completed the full vaccine series should catch up on missed shots.
Men ages 22 to 26 years:
- Those who have not received the vaccine in the past may still get the series of 3 shots. Talk to your provider.
- Those who have not finished the full vaccine series may catch up on missed shots. Talk to your provider.
- You should get the vaccine if you have sex with men.
- You should get the vaccine if your immune system is weak due to HIV, other conditions, or medication.
Women and men ages 27 to 45 years:
- Adults ages 27 through 45 may decide to get the HPV vaccine if they did not get vaccinated before.
- The decision to get the HPV vaccine, in this case, is based on discussions between the patient and the health care provider
- Because people in this age range have a higher likelihood of having been exposed to HPV already, the HPV vaccine may provide less benefit.
- People who received a dose of the vaccine and had an allergic reaction to it.
- Pregnant women.
- People who are ill with something more severe than a cold or have a fever should reschedule their vaccinations for after they have recovered.
The most common side effect of either vaccine is soreness at the injection site.
Meningococcal Disease
Meningococcal disease is caused by the meningococcal bacterium, Neisseria meningitidis (N meningitidis). The major, life-threatening meningococcal diseases are meningitis (infection of the lining of the brain and spinal cord) and septicemia (blood infection).
Meningococcus is spread when someone comes into contact with the respiratory and throat secretions of an infected person, such as through coughing or kissing.
Although just over 1,000 people a year get meningococcal disease in the United States, 10% to 15% of these people die. Of the people who survive, 11% to 19% have permanent health problems such as deafness and other nervous system problems.
People at highest risk of meningococcal disease include:
- Infants and children younger than 4 years of age
- Adolescents and young adults
- College freshmen living in dorms
- Children and adults who have certain medical problems, including weakened immune systems
- Children and adults who do not have normal spleen function
- Military recruits
- Researchers who work with N meningitidis.
- People who travel to areas of the world where there are high rates of meningococcal infection
MCV4
The meningococcal conjugate vaccine is called MCV4 for short because it protects against 4 types (strains) of N meningitidis. The vaccine is made from portions of N meningitidis bacteria.
MCV4 is a routine vaccine recommended for adolescents, 11 through 18 years old. High risk children may be vaccinated at age 10. One dose (shot) should be received at:
- 11 to 12 years old
- 16 years old (booster)
One or more doses of the vaccine are also recommended in certain high risk children and adults, ages 9 months to 55 years old.
MenB
The meningococcal serogroup B recombinant vaccine is another available vaccine that specifically protects against the most common form of meningococcus found in outbreaks, serotype B.
This vaccine is recommended for adolescents, greater than age 10 through age 25 years in certain high risk scenarios. Two doses should be given at least 1 month apart.
MPSV4
Another meningococcal vaccine, called Menomune, is a polysaccharide vaccine. It is the only meningococcal vaccine for people over age 55 years.
- People who received the vaccine and had allergic reactions to it.
- People with severe latex allergy should not receive Bexsero (a certain type of the type B vaccine).
- If you are pregnant or breastfeeding.
- People who are ill with something more severe than a cold or have a fever should reschedule their vaccinations for after they have recovered.
The most common side effect is soreness at the injection site or mild fever.
Rotavirus
Rotavirus is a virus that is the most common cause of diarrhea, cramps, and vomiting in infants and toddlers worldwide. The virus is spread from person to person. Before the vaccine was developed, as many as 80% of young children in the United States came down with symptoms of rotavirus disease. Tens of thousands of children were hospitalized and 20 to 60 children died of the disease each year.
The rotavirus vaccine is made from weakened, live rotavirus. It is an oral vaccine. Rotavirus vaccine is one of the recommended childhood immunizations. Depending on the vaccine brand, 2 or 3 doses are received. One dose is received at:
- 2 months old
- 4 months old
- 6 months old (if needed)
Babies with the following conditions should not receive the vaccine:
- Received a dose of the vaccine and had an allergic reaction to it
- Have a severe allergy to latex (only Rotarix contains latex; Rotateq is latex-free)
- Have an immune system problem
- Have had blocked intestine (intussusception)
- Babies who are severely ill, have a fever, or diarrhea or vomiting, should have their vaccinations rescheduled for after they have recovered.
Mild side effects that go away quickly include:
- Irritability
- Diarrhea
- Vomiting
Serious side effects may include blocked intestine (rare).
Other Vaccines
Many other vaccines are available, including vaccines for:
- Anthrax
- RSV (respiratory syncytial virus)
- Cholera
- Japanese encephalitis
- Rabies
- Smallpox
- Tuberculosis
- Typhoid fever
- Yellow fever
These vaccines are not routinely given to the general population. They are only recommended to people who are at risk of exposure to the specific germ. For more information about these vaccines, visit the CDC website -- www.cdc.gov/vaccines/vpd/vaccines-diseases.html.
Vaccines for Travelers to Developing Countries
People who are traveling to countries where certain infectious diseases are common should seek consultation in a travel clinic or check with the Centers for Disease Control and Prevention (www.cdc.gov/travel) for up-to-date information on immunization requirements for their destinations.
- All travelers should be up-to-date on any recommended vaccinations for childhood diseases, regardless of age. Booster shots may be required for travelers even if they have completed their initial series.
- Some countries require vaccinations against specific diseases such as yellow fever, meningitis, typhoid, cholera, Japanese encephalitis, and rabies under certain circumstances.
For more information, see the in-depth report on traveling to developing countries.
Resources
- CDC: Vaccines & Immunizations -- www.cdc.gov/vaccines/index.html
- Immunization Action Coalition -- www.immunize.org
- Institute for Vaccine Safety, Johns Hopkins School of Public Health -- www.vaccinesafety.edu
- National Vaccine Injury Compensation Program -- www.hrsa.gov/vaccine-compensation/index.html
- Vaccine Adverse Event Reporting System -- www.fda.gov/vaccines-blood-biologics/report-problem-center-biologics-evaluation-research/vaccine-adverse-events
- U.S. Department of Health and Human Services: Healthy People 2020. Immunization and infectious diseases -- www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases
References
Bernstein HK, Kilinksy A, Orenstein WA. Immunization practices. In: Kliegman RM, St. Geme JW, Blum NJ, Shah SS, Tasker RC, Wilson KM, eds. Nelson Textbook of Pediatrics. 21th ed. Philadelphia, PA: Elsevier; 2020:chap 197.
Centers for Disease Control and Prevention website. Recommended immunization schedule for adults aged 19 years or older, United States, 2020. www.cdc.gov/vaccines/schedules/hcp/imz/adult.html. Updated February 3, 2020. Accessed May 20, 2020.
Centers for Disease Control and Prevention website. Recommended immunization schedule for children and adolescents aged 18 years or younger, United States, 2020. www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Updated February 3, 2020. Accessed May 20, 2020.
Dormitzer PR. Rotaviruses. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 9th ed. Philadelphia, PA: Elsevier; 2020:chap 150.
Freedman MS, Hunter P, Ault K, Kroger A. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older — United States, 2020. MMWR Morb Mortal Wkly Rep 2020;69:133–135. PMID: 32027627 pubmed.ncbi.nlm.nih.gov/32027627.
Freedman DO. Protection of travelers. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Philadelphia, PA: Elsevier; 2020:chap 318.
Kroger AT, Pickering LK, Mawle A, Hinman AR, Orenstein WA. Immunization. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 9th ed. Philadelphia, PA: Elsevier; 2020:chap 316.
Robinson CL, Bernstein H, Poehling K, Romero JR, Szilagyi P. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger — United States, 2020. MMWR Morb Mortal Wkly Rep 2020;69:130–132. PMID: 32027628 pubmed.ncbi.nlm.nih.gov/32027628.
Reviewed By: Charles I. Schwartz MD, FAAP, Clinical Assistant Professor of Pediatrics, Perelman School of Medicine at the University of Pennsylvania, General Pediatrician at PennCare for Kids, Phoenixville, PA. Also reviewed by David Zieve, MD, MHA, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team.