Prostate cancer - InDepth
Highlights
Prostate Cancer Screening Guidelines
The United States Preventive Services Task Force (USPSTF) recommends men aged 55 to 69 years consider prostate cancer screening based on shared decision making with their clinician. Screening may provide a small reduction in the risk of death from prostate cancer in some men. However, the USPSTF cites concerns regarding potential harms from screening including false positives, overtreatment, and complications from unnecessary treatment.
The American Cancer Society's guidelines for early detection of prostate cancer recommend that men discuss with their doctors the uncertainties, risks, and potential benefits of screening for prostate cancer before deciding whether to be tested. For men who are interested, screening should be started based on risk of developing cancer. Those at average risk start at 50, but those at higher risk start earlier. The American Urological Association and the American College of Physicians have similar guidelines, including shared decision making and age-based testing depending on the man's risk of developing prostate cancer.
In general, the current consensus is that there is no "one size fits all" guideline for who should receive prostate cancer screening and at what age. It is important to discuss with your doctor your questions and concerns regarding prostate cancer screening.
Follow-Up Care Guidelines
The American Cancer Society has guidelines for the long-term care of prostate cancer survivors. The guidelines recommend:
- Coordinating care between your oncologist and primary care doctor.
- Engaging in healthy lifestyle by exercising regularly and eating nutritious foods.
- Having follow-up tests for PSA levels (every 6 to 12 months for the first 5 years after treatment, and once a year after that), and an annual digital rectal exam.
- Monitoring side effects of cancer treatment including physical (urinary, bowel, and sexual function) and emotional (depression, anxiety).
Supplements Harmful, Not Protective
The most recent analysis from the Selenium and Vitamin E Prevention Trial (SELECT) confirms that selenium and vitamin E supplements do not prevent prostate cancer and can actually increase the risk of developing it.
Introduction
Prostate cancer is a malignant tumor that originates in the prostate gland. As with any cancer, if it advances or is left untreated in early stages, it may eventually spread through the blood and lymph fluid to other organs. Fortunately, prostate cancers tend to be relatively slow growing compared to other cancers. Most older men eventually develop at least microscopic evidence of prostate cancer, but it often grows so slowly that many men with prostate cancer "die with it, rather than from it."

The prostate gland is an organ that surrounds the urinary urethra in men. It secretes fluid that mixes with sperm to make semen.
Description of the Prostate Gland
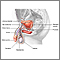
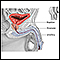
The prostate is a walnut-shaped gland located below the bladder and in front of the rectum. It wraps around the urethra (the tube that carries urine from the bladder and through the penis). The central area of the prostate that wraps around the urethra is called the transition zone. The entire prostate gland is surrounded by a dense, fibrous capsule.
Functions of the Prostate Gland
The prostate gland provides the following functions:
- The glandular cells produce a milky fluid. During sexual intercourse, the smooth muscles contract and squeeze this fluid into the urethra. Here, it mixes with sperm and other fluids to make semen.
- The prostate gland also contains an enzyme, called 5 alpha-reductase, which converts testosterone to dihydrotestosterone, another male hormone that has a major impact on the prostate.
Changes During the Lifespan
The prostate gland undergoes many changes during the course of a man's life. At birth, the prostate is about the size of a pea. It grows only slightly until puberty, when it begins to enlarge rapidly, attaining normal adult size and shape (about that of a walnut) when a man reaches his early 20s. The gland generally remains stable until men reach their mid-40s, when, in most men, the prostate begins to enlarge again through a process of cell multiplication.
Risk Factors
The major risk factors for prostate cancer are age, family history, and ethnicity.
Age
Prostate cancer occurs almost exclusively in men over age 40 and most often after age 50. The average age at diagnosis is about 67. By age 70, about two thirds of men have at least microscopic evidence of prostate cancer. Fortunately, the cancer is usually very slow growing, and older men with the cancer typically die of something else, often without even knowing they technically have prostate cancer.
Family History and Genetic Factors
Heredity plays a role in some types of prostate cancers. Men with a family history of the disease have a higher risk of developing prostate cancer. Having one family member with prostate cancer doubles a man's own risk, and having three family members exponentially increases risk. A specific gene, named HPC1 (hereditary prostate cancer-1) was the first of several genes linked to inherited types of the disease. Carriers of one of the genes commonly associated with breast cancer, BRCA2, appear to have an increased risk for prostate cancer. Other inherited cancer syndromes may be associated with an increased risk of prostate cancer also. Exact role of genetic testing to screen men's prostate cancer risk is not clear.
Scientists are researching other genetic variations that may increase prostate cancer risk.
Race and Ethnicity
In the US, African American men have higher rates of prostate cancer than men of other races. They are also more likely to develop prostate cancer at a younger age and to have more aggressive forms of the disease.
Worldwide, prostate cancer is more common in North America and northern Europe and less common in Africa, Latin America, and Asia. Diet and other factors may play a role. For example, Asians who live in the United States have a higher rate of prostate cancer than those who live in Asia.
Hormones
Male hormones (androgens), particularly testosterone, may play a role in the development or aggressiveness of prostate cancer. Other types of hormones, such as the growth hormone insulin-like growth factor-1 (IGF-1), may also be associated with some types of prostate cancer.
Inflammation and Infection
Prostatitis (inflammation of the prostate gland) may possibly be associated with increased prostate cancer risk. There may also be a possible relationship between prostate cancer and sexually transmitted infections, such as herpes virus and human papillomavirus, but no definite association has yet been proven.
Dietary Factors
Because a Western lifestyle is associated with prostate cancer, dietary factors have been intensively studied. Results have been inconsistent and inconclusive, however.
Fats
Some studies have found an association between high fat intake and prostate cancer. In particular, high consumption of red meat and high-fat dairy products has been linked to increased risk for prostate cancer. (Some evidence suggests that the calcium consumed in low- and high-fat dairy products may increase risk.) In contrast, the omega-3 fats found in certain fish (salmon, sardines, fresh tuna) may possibly be protective.
Vegetables and Fruits
A diet rich in vegetables, fruits, and legumes appears to protect against prostate cancer. However, it is not clear whether this is due to the nutrients contained in these foods, or the fact that these foods are low in fat. No specific vegetable or fruit has been proven to decrease risk. Lycopene, which is found in tomatoes, has been a target of research interest, but the evidence for its protective benefit is inconclusive.
Vitamins and Minerals
Nutritious foods that are part of a healthy diet are the best sources for vitamins and minerals, not supplement pills. Major clinical studies have found that vitamin, multivitamin, and mineral supplements do not prevent prostate cancer. Selenium (a mineral) and vitamin E have been the most extensively studied.
Data from the largest study, the Selenium and Vitamin E Prevention Trial (SELECT), indicate that neither selenium nor vitamin E, whether taken separately or together, help prevent prostate cancer. In fact, these supplements could actually increase risk for this cancer.
The most recent analyses from SELECT suggest that men who have high natural levels of selenium may double their risk for aggressive prostate cancer by taking selenium supplements. Men who have low levels of selenium in their body, could increase their risks for all grades of prostate cancer by taking vitamin E.
Possible Preventive Factors
The below factors may help reduce the risk of developing prostate cancer.
Diet
Eat a healthy diet rich in fruits, vegetables, and legumes (beans). Limit consumption of red meat and high-fat dairy products. There is some evidence that a low-fat diet may help protect against prostate cancer.
Weight and Exercise
Achieve and maintain a healthy weight through a nutritious diet and regular physical activity.
5-ARIs (Controversial)
If you are at higher-than-average risk for prostate cancer, you may wish to discuss with your doctor whether 5-ARI medications may help lower your risk. The 5-ARI drugs finasteride (Proscar, generic) and dutasteride (Avodart, generic) are prescribed to help improve urinary symptoms associated with benign prostatic hyperplasia (BPH). Studies have suggested that 5-ARIs lower a man's overall risk for developing prostate cancer.
However, the FDA advised in the past that these drugs may increase the risk of developing high-grade aggressive types of prostate cancer. More recent evidence does not indicate an increased risk for cancer with these medicines. They should not be used for prostate cancer prevention. The 5-ARIs are not approved for prostate cancer prevention and their use for this purpose is controversial.
Prognosis
Prostate cancer is the most common internal cancer in American men. (For men, skin cancer is the most common cancer, and only lung cancer causes more cancer deaths.) About 1 in 7 men will be diagnosed with prostate cancer over the course of their life. But because so many prostate tumors are low-grade and slow growing, and men are usually older when they are diagnosed with it, most men diagnosed with prostate cancer eventually die of something else.
At the time of diagnosis, most men have localized prostate cancer (cancer confined to the prostate gland). The prognosis for men with localized prostate cancer is excellent. Nearly 100% of men with localized prostate cancer live at least 5 years after diagnosis. The same is true for men with regional prostate cancer, which means the cancer has spread from the prostate gland to only nearby areas in the body.
Five percent of men are diagnosed with advanced or distant cancer that has spread throughout the body. For these men, the 5-year relative survival rate is 29%.
A survival rate indicates the percentage of patients who live a specific number of years after the cancer is diagnosed. A relative survival rate compares the survival of people with a specific type of cancer to the expected survival of people who do not have cancer and will die from other causes.
Overall, for prostate cancer, the 10-year relative survival rate is about 99% and the 15-year survival rate is about 94%. After 15 years, survival rates stabilize.
The odds of survival depend in part on how advanced the cancer is when a man is first diagnosed, as well as changes identified when tissue is examined under the microscope. Men who are diagnosed with low-grade prostate cancers have a minimal risk of dying from prostate cancer for up to 20 years after diagnosis. However, men diagnosed with more aggressive forms of prostate cancer have a higher risk of dying within 10 years.
If cancer recurs after initial treatment for early-stage tumors, it is still potentially curable if it is contained within the prostate, although in most cases the cancer will have spread. Hormone treatments for such recurring cancers can often prolong survival for years, although the cancer will almost always return.
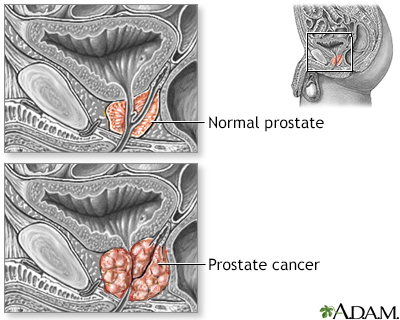
The treatment of prostate cancer varies depending on the stage of the cancer and may include surgical removal, radiation, chemotherapy, hormonal manipulation or a combination of these treatments.
Symptoms
Prostate cancer usually causes no symptoms in the early stages. As the malignancy spreads, it may constrict the urethra and cause urinary problems.
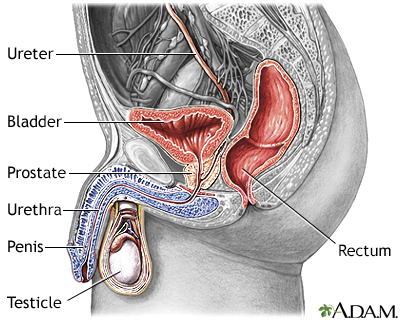
Urine flows from the kidney through the ureters into the urinary bladder where it is temporarily stored. As the bladder becomes distended with urine, nerve impulses from the bladder signal the brain that it is full, giving the individual the urge to void. By voluntarily relaxing the sphincter muscle around the urethra, the bladder can be emptied of urine. Urine then flows out through the urethra.
Later Stage Urinary Symptoms
Later-stage urinary symptoms may include:
- Weak urinary stream
- Inability to urinate
- Blood in the urine
- Interruption of urinary stream (stopping and starting)
- Frequent urination (largely at night)
- Pain or burning during urination
Although advanced prostate cancer can cause these symptoms, they are more commonly caused by benign prostatic hyperplasia and other non-cancerous conditions.
Late Stage General Symptoms
Significant pain in one or more bones may indicate the occurrence of metastases (spreading of cancer) to the bones. This chronic pain occurs most often in the spine and sometimes flares in the pelvis, the lower back, the hips, or the bones of the upper legs. It may be accompanied by significant unexplained weight loss and fatigue.
Conditions with Similar Symptoms
(BPH)
BPH is a urinary condition that can develop into an enlarged prostate, which puts pressure on the urethra and causes urinary problems. BPH is not a cancerous or precancerous condition, but its symptoms can mimic late-stage prostate cancer.
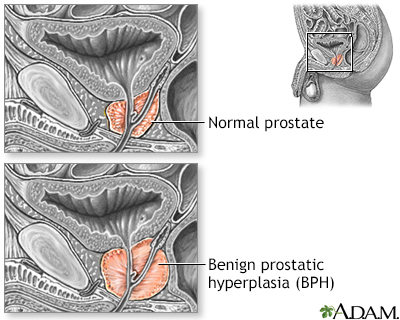
Benign prostatic hypertrophy (BPH) is a non-cancerous enlargement of the prostate gland, commonly found in men over the age of 50.
Prostatitis
Prostatitis is an inflammation of the prostate, often caused by bacterial infection. Symptoms include urgency, frequency, and pain in urination, sometimes accompanied by fever or blood in the urine.
Diagnosis
Screening for Prostate Cancer
The goal of screening is to find cancer at an early stage before it has caused symptoms. To be considered successful, a screening test should lead to treatments that prolong life or reduce discomfort and improve the quality of life.
Two standard screening tests are used for early detection of prostate cancer:
PSA test
The PSA blood test measures the blood level of a protein called prostate-specific antigen. Prostate specific antigen is a protein produced in the prostate gland that keeps semen in liquid form. Prostate cancer cells appear to produce this protein in elevated quantities.
The PSA protein exists in the body both as a free form and also bound to a larger molecule. The free PSA test measures the percent of PSA that is free or unbound in the body. The lower the ratio or percentage of free PSA, the more likely cancer is present. A possible use of this test is when deciding whether to pursue a biopsy for men with PSA levels between 4 and 10 ng/mL (nanograms/mL) or 4 and 10 mcg/L (micrograms/L), especially in those at the older age range. A result of 25% or lower indicates a higher risk of cancer. However, the test is not perfect and men with a free PSA above 25% may still have cancer. As a result, the exact role of this test in decision making is unclear.
Digital rectal examination (DRE)
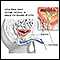
The DRE is a physical examination. The doctor inserts a gloved and lubricated finger into the patient's rectum and feels the prostate for bumps or other abnormalities.
Controversies of screening for prostate cancer
There is uncertainty and controversy over whether the benefits of regular screening for prostate cancer outweigh the risks for most men. Prostate cancer is very common, largely among older men, and is often slow growing. Doctors cannot yet predict accurately which early-stage tumors pose a risk of being aggressive and need treatment, and which tumors should be left alone.
The concern is that routine screening for early detection of tumors may lead to invasive and unnecessary treatment for men who would otherwise die from other causes. The general consensus is that screening should be an individual’s decision based on personal preferences and risk of developing prostate cancer.
The controversy over prostate cancer screening centers on the use of the PSA test as a screening tool. This test by itself is not accurate enough to either rule out or confirm the presence of cancer. Although PSA levels are often elevated in men with prostate cancer, some men with prostate cancer have normal PSA results. In addition, PSA levels can be increased by various factors other than prostate cancer, including benign prostatic hyperplasia, prostatitis, advanced age, and ejaculation within 48 hours of the test.
The main concern is that PSA screening may result in the detection of some cancers that would never have bothered the patient and would never have posed a threat to his life. Older men are less likely to die from prostate cancer than from heart disease and other problems. Relying too much on the test may lead to unnecessary biopsies and potentially harmful treatments. Several major studies have found that PSA screening saves few, if any, lives. On the other hand, there is a small potential of reducing the chance of death from prostate cancer in some cases. Therefore, for men aged 55 to 69, the decision to have a PSA test should be taken on an individual basis, considering risk factors and personal preference. For men 70 years and older, the United States Preventive Services Task Force (USPSTF) currently recommends against PSA-based screening for prostate cancer.
The American Cancer Society (ACS) recommends that starting at age 50, men with average risk for prostate cancer should discuss with their doctors the pros and cons of having a PSA test with or without a digital rectal exam. Men who are at higher-than-average risk for prostate cancer (including those with a family history of prostate cancer and all African Americans) should initiate this talk at age 40 to 45. When and how often a man should be retested depends on his PSA level. The American Urological Association (AUS) recommends the test primarily for men ages 55 to 69 years, after a similar discussion of risks and benefits, with retesting every 2 years.

Prostate cancer is the most common internal cancer in men in the United States. Prostate cancer forms in the prostate gland, and can sometimes be felt on digital rectal examination. This is one of the purposes of the digital rectal exam.
Tests to Diagnose Prostate Cancer
Biopsy
If cancer is suspected, the doctor will order a biopsy. Only a biopsy, in which a tiny sample of prostate tissue is surgically removed, can actually confirm a diagnosis of prostate cancer. A biopsy is usually performed to confirm or rule out cancer based on a combination of PSA test levels, findings on the DRE, family history, and patient's age and ethnicity. If a biopsy gives a negative result but the doctor still suspects cancer, repeat biopsies may be performed.
An ultrasound procedure called transrectal ultrasonography (TRUS) may be used to help the doctor see where to take the needle biopsy. Ultrasound is not effective as a diagnostic tool by itself because it cannot differentiate very well between benign inflammation and cancer.
Tests after Cancer is Diagnosed
PSA Levels and PSA Velocity
Once cancer is diagnosed, PSA levels may help to determine its extent. If PSA levels are lower than 20 ng/mL (20 mcg/L), it is likely that the cancer has not spread to distant sites. PSA levels over 40 ng/mL (40 mcg/L) are a strong indicator that cancer has metastasized (spread elsewhere in the body).
PSA levels are monitored after treatments begin. Changes in the level can show if a treatment is working or if the cancer has come back. The American Cancer Society recommends monitoring PSA levels every 6 to 12 months for the first 5 years after treatment ends, and annually after that.
Doctors also monitor how quickly PSA levels rise over time. This rate is called PSA velocity (PSAV). The PSAV may help determine when treatment should begin and which treatment should be used. A high rate of PSAV is considered to be 2 ng/mL (2 mcg/L) a year. Research suggests that men with early-stage prostate cancer who have a slow PSAV are more likely to live longer than men with rapidly rising PSA levels.
Test for Metastasis
If the biopsy indicates cancer, and the PSA is above 20 ng/mL (20 mcg/L), the doctor will order other tests to determine whether or how far the cancer has spread:
- Bone scans and x-rays may reveal whether the cancer has invaded the bones. To perform a bone scan, the doctor injects a low dose of a radioactive substance into the patient's vein, which accumulates in bones that have been damaged by cancer. A scanner then reveals how much of the radioactive material has accumulated.
- Computed tomography (CT) or magnetic resonance imaging (MRI) scans can further pinpoint the location of cancer that has spread beyond the prostate.
Genetic Tests
Genetic tests may help predict if a cancer is low-risk or if it could become aggressive (fast-growing). Genetic tests measure the gene activity in prostate tissue tumor samples and can help predict whether the cancer will grow and spread.
Tests like these may help doctors determine whether or not a man with prostate cancer needs treatment, and could potentially spare low-risk patients from treatments that may do more harm than good. However, genetic tests are expensive, their clinical use and overall benefit has not yet been established, and they may not be covered by all insurance plans.
Staging and Grading
Grading refers to how abnormal the cells look under a microscope and how likely the cancer is to advance and spread. A pathologist will read the biopsy report and assign a grade to the tumor cells using the Gleason system of scoring.
Staging refers to the extent the cancer has spread in the body. A doctor stages prostate cancer based on the Gleason grade, TNM staging system, PSA test result, digital rectal exam, and possibly imaging tests. The stage of cancer helps determine treatment options.
The Gleason Grading System
The Gleason grading system refers to how abnormal your prostate cancer cells look and how likely the cancer is to advance and spread. A lower Gleason grade means that the cancer is slower growing and not as aggressive.
Gleason Score
The first step in determining the Gleason grade is to determine the Gleason score.
- When looking at cells under the microscope, the doctor assigns a number (or grade) to the prostate cancer cells between 1 and 5.
- This grade is based on how abnormal the cells appear. Grade 1 means that the cells look like normal prostate cells. Grade 5 means that the cells look very different from normal prostate cells.
- Most prostate cancers contain cells that are different grades. So the two most common grades are used.
- The Gleason score is determined by adding the two most common grades. For example, the most common grade of the cells in a tissue sample may be grade 3 cells, followed by grade 4 cells. The Gleason score for this sample would be 7.
Higher numbers indicate a faster growing cancer that is more likely to spread.
Currently, the lowest score assigned to a tumor is grade 6. Scores below 6 show normal to near-normal cells. Most cancers have a Gleason score of between 6 and 7.
Gleason Grading System
Sometimes, it can be hard to predict how well patients will do based just on their Gleason scores alone.
- For example, your tumor may be assigned a Gleason score of 7 if the two most common grades were 3 and 4. The 7 may come either from adding 3 + 4 or from adding 4 + 3.
- Overall, someone with a Gleason score of 7 that comes from adding 3 + 4 is felt to have a less aggressive cancer than someone with a Gleason score of 7 that comes from adding 4 + 3. That is because the person with a 4 + 3 =7 grade has more grade 4 cells than grade 3 cells. Grade 4 cells are more abnormal and more likely to spread than grade 3 cells.
A new 5 Grade Group System has recently been created. This system does a better job of describing how a cancer will behave and respond to treatment.
- Grade group 1: Gleason score 6 or lower (low-grade cancer)
- Grade group 2: Gleason score 3 + 4 = 7 (medium-grade cancer)
- Grade group 3: Gleason score 4 + 3 = 7 (medium-grade cancer)
- Grade group 4: Gleason score 8 (high-grade cancer)
- Grade group 5: Gleason score 9 to 10 (high-grade cancer)
A lower group indicates a better chance for successful treatment than a higher group. A higher group means that more of the cancer cells look different from normal cells. A higher group also means that it is more likely that the tumor will spread aggressively.
TNM Staging System
A tumor's stage is an indication of how far it has spread from its original site. Cancers are staged according to whether they are still localized (still within the prostate gland) or have spread beyond the original site. The current prostate cancer staging system is the TNM system.
The TNM system refers to clinical tumor stages as:
T Stages
T is for tumor. T followed by numbers 0 through 4 refers to the size and extent of the tumor itself.
N Stages
N is for nodes. N followed by 0 through 3 refers to whether the cancer has reached the regional lymph nodes, which are located next to the prostate in the pelvic region.
M Stages
M is for metastasis (tumors developing outside the prostate).
The T, N, and M stages are used along with the grade, PSA test result, and other factors to determine the overall stage of the cancer.
The higher the stage, the more advanced the cancer.
Stage I cancer
The cancer is found in only one part of the prostate. Stage I is called localized prostate cancer. It cannot be felt during a DRE or seen with imaging tests. If the PSA is less than 10 and the Gleason score is 6 or less, Stage I cancer is likely to grow slowly.
Stage II cancer
The cancer is more advanced than stage I. It has not spread beyond the prostate and is still called localized. The cells are less normal than cells in stage I, and may grow more rapidly. There are two types of stage II prostate cancers:
- Stage IIA is most likely found in only one side of the prostate.
- Stage IIB may be found in both sides of the prostate.
Stage III cancer
The cancer has spread outside the prostate into local tissue. It may have spread into the seminal vesicles. These are the glands that make semen. Stage III is called locally advanced prostate cancer.
Stage IV cancer
The cancer has spread to distant parts of the body. It could be in nearby lymph nodes or bones, most often of the pelvis or spine. Other organs such as bladder, liver, or lungs can be involved.
[For more information on staging, see Treatment section of this report.]
Treatment
Treatment choices are generally based on the person's age, the stage and grade of the cancer, overall health status, and the person's personal preferences for the risks and benefits of each therapy.
People should be aware that doctors may prefer a specific treatment depending on their specialty, with urologists and medical oncologists tending to recommend watchful waiting, surgery, or hormone therapy and radiation oncologists recommending radiation therapy. It is always wise to seek a second opinion to feel comfortable about proceeding with a treatment program. Delaying treatment, while having the cancer monitored for signs of progression (watchful waiting or active surveillance), is also an acceptable option for many people.
Depending on the cancer stage and other factors, patients have 4 main treatment options that are commonly used:
Active surveillance
, also called watchful waiting, involves monitoring the tumor for cancer progression to determine if and when treatment should be started.Surgery (radical prostatectomy)
removes the prostate gland. The vessels that carry semen and surrounding tissue may also be removed. Radical prostatectomy may be performed either through open surgery or using laparoscopic or robotic techniques.Radiation therapy
targets the tumor either externally (external beam radiation) or internally (implanted "seeds").Androgen deprivation therapy
, also called hormone therapy, uses orchiectomy (surgical removal of the testicles) or drugs to stop production of male hormones.
It is important to understand that there are few or no high-quality trials comparing the different treatment approaches with each other.
Stage I and Stage II Treatment Options (Localized Prostate Cancer)
Localized cancer is classified as low, intermediate, or high risk. A higher risk indicates that the tumor is more likely to be aggressive. Doctors determine the risk category by using criteria such as PSA levels, Gleason score, the clinical stage of the tumor, and patient age.
Active surveillance for treating localized prostate cancer:
- Considered a good option for people with localized, low-risk prostate cancer.
- Best candidates for this approach are men over age 65, who also have PSA levels below 10 ng/mL (10 mcg/L), a Gleason score of 6, and no sign of tumor spread outside of the prostate.
Radical prostatectomy for localized prostate cancer:
- Prostatectomy is considered an option for Stage I or II who chose not to do active surveillance.
- Compared with watchful waiting, radical prostatectomy appears to lower the risk of cancer recurrence and death, at least in men younger than age 65 at the time of diagnosis.
- Removal of regional pelvic lymph nodes is not needed for lower risk prostate cancer, but is more likely to be done for medium or higher cancer.
- Prostatectomy may produce better survival rates than radiotherapy as primary treatment. This benefit is more likely for younger men with intermediate or high-risk localized prostate cancer.
External beam radiation therapy for localized prostate cancer:
- The best candidates to use radiation therapy as the primary or first treatment are men over age 65, who also have PSA levels below 10 ng/mL (10 mcg/L), a Gleason score of 6, and no sign of tumor spread outside of the prostate.
- For men with intermediate or high risk localized prostate cancer, radiation is most often not used as the primary therapy. However, radiation treatment may also be given after prostatectomy for these people.
Implant radiation therapy (brachytherapy) for localized prostate cancer:
- Brachytherapy is a procedure to implant radioactive seeds (pellets) into the prostate gland to kill prostate cancer cells. The seeds may give off high or low amounts of radiation.
- Brachytherapy is often used for men with a small prostate cancer that is found early and is slow-growing.
Hormonal/androgen deprivation therapy for localized prostate cancer:
- Hormonal therapy as the initial treatment is seldom recommended for localized prostate cancer. It may be used to relieve symptoms in patients with poor prognosis.
- For men at intermediate and high risk, adding androgen deprivation therapy to external beam radiation may improve survival but also increases adverse side effects. These include diabetes, anemia, osteoporosis, and heart disease.
- Adding hormonal therapy to radical prostatectomy does not appear to improve survival or cancer recurrence rates.
Cryotherapy or high-intensity focused ultrasound (HIFU) are other possible treatments for localized prostate cancer. However, the evidence to support these treatments as first-line therapy is not as strong.
Stage III Treatment Options (Locally Advanced Cancer)
Stage III prostate cancer is present if the tumor has spread beyond the capsule of the prostate gland, but not to the regional lymph nodes or elsewhere in the body. A diagnosis of Stage III prostate cancer:
- May be made before any primary treatment has begun.
- May only be discovered after prostatectomy done for what is thought to be localized prostate cancer (Stage I or II).
Treatment for Stage III prostate cancer may include:
- External beam radiation with or without androgen deprivation therapy (hormone therapy).
- Brachytherapy may be done for an extra boost of radiation after external beam radiation is completed.
- Radical prostatectomy with pelvic lymph node removal as the first therapy is another treatment option.
- Hormonal therapy (androgen deprivation therapy) is often used after radiation therapy or to relieve symptoms.
Stage IV Treatment Options (Advanced Cancer)
- Androgen deprivation therapy is most often the first treatment for people with Stage IV prostate cancer.
- Chemotherapy may also be tried for those with significant metastatic disease, using the drug docetaxel as first-line therapy in addition to androgen deprivation therapy.
- External beam radiation therapy with or without androgen deprivation therapy may be used to relieve symptoms in the pelvis or bones.
Recurrent or Persistent Prostate Cancer
If prostate cancer has been treated with radical prostatectomy, PSA levels should drop to zero after surgery. After radiation, PSA levels do not drop as far because some of the normal prostate gland remains. A sudden rise or persistently elevated PSA levels after treatment are often indications that prostate cancer persists.
It is common for PSA levels to temporarily rise or "bounce" following radiation seed implantation without representing true cancer recurrence. Rising PSA levels do not necessarily mean that the cancer has spread or even that clinical cancer will recur during a man's lifetime.
Treatment options for recurrent cancer depend on various factors, including prior treatment, site of recurrence, coexistent illnesses, and individual patient considerations. For men with:
- Cancer that recurs locally after prostatectomy. Radiation therapy, androgen-deprivation therapy (hormone therapy).
- Cancer that recurs locally after radiation therapy. Androgen deprivation therapy, prostatectomy (very select patients), cryosurgery.
- Recurrent cancer that has spread (metastasized). Androgen-deprivation therapy (either medical or surgical).
Unfortunately, for some prostate cancers that are more aggressive, treatment with hormone therapy and chemotherapy may no longer work. The PSA may begin to rise and new evidence of spreading cancer may be present.
These people are considered to have what is called castration resistance. Their treatment options at this point include continuation of androgen-deprivation therapy plus one of these treatments:
- Apalutamide (Erleada) for people without metastases.
- Abiraterone (Zytiga) plus prednisone.
- Enzalutamide (Xtandi).
- Radium-223 (Xofigo) for people primarily with bone metastases.
- Chemotherapy (most commonly docetaxel).
- Sipuleucel-T (Provenge) for select patients. This treatment is a form of immunotherapy.
Palliative care is an important consideration for all people at this stage of disease.
Comparing Side Effects of Treatments
Prostate cancer treatments can cause distressing side effects including erectile dysfunction, urinary and bowel leakage (incontinence), and fatigue. A man must weigh his own emotional responses to the possibility of these side effects versus the possible stress of active surveillance.
Side effects vary among people and it is difficult to predict how an individual patient will respond. In general, the side effects most likely to occur by treatment modality are:
- Prostate removal surgery (prostatectomy) is more likely to cause urinary incontinence and erectile dysfunction than radiation therapy (radiotherapy). Nerve-sparing prostatectomy produces better sexual function than conventional radical prostatectomy.
- External beam radiation therapy produces better urinary control and sexual function than brachytherapy (internal radiation). Brachytherapy has better results for these side effects than radical prostatectomy.
Radiotherapy (both brachytherapy and external beam radiation) generally causes more bowel problems than surgery. These differences in side effects are most apparent in the short-term (2 to 5 years after treatment). In the long-term (15 years after treatment), most men treated with either surgery or radiation experience reduced sexual and urinary function, but this may be due in part to advancing age as well.
Follow-Up After Treatment
The American Cancer Society's guidelines for long-term care of prostate cancer survivors recommend:
Coordinate care with your doctors.
Ask your oncologist for a written follow-up care plan that you can share with your primary care doctor. The plan should outline what types of medical issues each doctor will oversee during your future care.Engage in healthy behaviors.
Nutritious eating and regular physical activity can reduce the chances for cancer recurrence. Maintain a healthy weight, exercise regularly, limit alcohol consumption to no more than 2 drinks a day, and do not smoke. Consume plenty of fruits, vegetables, and whole grains in your diet, and limit saturated fats.Get necessary tests for cancer.
Prostate cancer survivors should have a PSA test every 6 to 12 months for the first 5 years after treatment ends, and once every year after that. They should also have an annual digital rectal exam. Men treated with radiotherapy may be at increased risk for bladder or colorectal cancer and need other types of screening tests.Monitor side effects of treatment.
Depending on your cancer treatment, you may need other testing. For example, patients treated with androgen deprivation therapy are at increased risk for anemia and need to have annual blood count tests. Your doctor should assess the physical side effects of your treatment (urinary, bowel, erectile dysfunction) as well as your emotional well-being.Report symptoms and discuss concerns.
Discuss with your doctor any concerns you have about depression, anxiety, or sexual function. Let your doctor know about any new symptoms you experience such as blood in the urine or pain.
Active Surveillance (Watchful Waiting)
Active surveillance, also called watchful waiting, involves lifestyle change and careful monitoring of cancer with conversion to active therapy if the disease progresses. With this approach, people have a digital rectal exam and PSA blood test about every 3 to 6 months. They may also need regular ultrasounds and prostate biopsies. If test results indicate cancer progression, doctor and patient then consider treatment options (surgery, radiation, or drugs).
People should also engage in lifestyle changes with regular physical exercise and healthy diet. They should immediately report to their doctors any new symptoms such as weight loss, pain, urinary problems, or fatigue.
Active surveillance is usually reserved for older men (over age 65) who have relatively low PSA levels (below 10 ng/mL or 10 mcg/L) and tumors that have been classified as low-risk based on staging and Gleason score. More aggressive treatments (surgery and radiation) are usually recommended for younger men or those with higher-risk cancer. The general recommendation is that aggressive therapy is suitable for those who have a life expectancy of more than 10 years and who have localized but mid- to high-grade tumors.
Some doctors think that because prostate cancer grows so slowly, it is likely that older men will die from causes unrelated to the cancer. There is therefore little potential benefit from surgery or radiation, with both posing a risk for erectile dysfunction and incontinence. However, delaying treatment may allow the cancer to grow and spread. The choice is a difficult one. It is important that people find a doctor who can provide them with all the necessary information so that they can make an informed decision.
Surgery
In men whose cancer is confined to the prostate (localized cancer), surgical resection (radical prostatectomy) offers the potential for cure. Most people can consider themselves disease-free if their PSA levels remain undetectable 10 years after surgery.
Radical Prostatectomy
Radical prostatectomy is the surgical removal of the entire prostate gland along with the seminal vesicles (the vessels that carry semen) and surrounding tissue. The surgeon may also remove the pelvic lymph nodes (a procedure called pelvic lymphadenectomy). The cut can be made in one of the following regions:
- Retropubically (through the abdomen and under the pubic bone, exposing the entire surface of the prostate). This is the approach used most often.
- Through the perineum (the area between the scrotum and the anus). The perineal approach causes less bleeding and has a shorter recovery time, but it makes it more difficult to preserve nerves and remove lymph nodes. This approach is now rare.
The gland and other structures are then removed. The operation lasts 2 to 4 hours.
Minimally Invasive Prostatectomy
Less invasive surgical techniques with laparoscopy use smaller cuts and allow faster recovery, but they require special surgical training. Laparoscopic surgery inserts an instrument with a small video camera attached to it to help guide the surgeon.
Robotic-assisted laparoscopic radical prostatectomy involves the surgeon directing a robotic arm through a computer monitor. Not every hospital can do robotic-assisted laparoscopic prostatectomy, and these procedures are difficult to perform. Although robotic surgery is gaining popularity, it is not clear that it produces better results than traditional operations.
Nerve-Sparing Techniques
In retropubic open surgery, laparoscopic surgery, and robot-assisted surgery, the surgeon will attempt to spare the nerves that control erection:
- A bilateral nerve-sparing procedure saves the nerves on both sides of the sex organs.
- A unilateral procedure saves nerves on only one side.
Nerve-sparing techniques can improve quality of life, by decreasing the occurrence of incontinence and erectile dysfunction. In cases where the tumor lies too close to the nerves, nerve-sparing techniques may not be possible.
Recuperation
People remain hospitalized for about 3 days after an open procedure or 2 days after less invasive procedure. Full recovery at home takes about 3 to 5 weeks. A temporary catheter used to pass urine is kept in place when the person is sent home and is usually removed about 3 weeks after the open operation or 1 week after a minimally invasive procedure. In general, younger people with early-stage cancers recover fastest and experience the fewest side effects.
Complications from Radical Prostatectomy
The main complications from radical prostatectomy are urinary incontinence and erectile dysfunction. Other complications include the usual risks of any surgery, such as blood clots, heart problems, infection, and bleeding. Minimally invasive procedures usually result in less pain and a faster return to activity.
Urinary Incontinence
Urinary incontinence is a common complication. When the urinary catheter is first removed following surgery, nearly all patients lack control of urinary function and will leak urine for at least a few days and sometimes for months. Normal urinary function often returns within about 18 months. A percentage of men will continue to have small amounts of leakage with heavier exertion or possibly sexual activity.
If incontinence persists beyond a year, patients may require drug therapy or surgery.
Erectile Dysfunction
Erectile dysfunction after radical prostatectomy is caused by nerves that were damaged or removed during the surgery. Virtually all men will have problems with erectile function after surgery. It can take up to 1 to 2 years to recover erectile function after surgery. Because seminal glands are removed along with the prostate gland during surgery, men who regain sexual function will not produce semen during orgasm ("dry ejaculation").
With the use of nerve-sparing techniques, men below age 60 who were sexually active before surgery seem to have a better chance of preserving sexual function. PDE5 inhibitor drugs such as sildenafil (Viagra), vardenafil (Levitra), tadalafil (Cialis), or avanafil (Stendra) may help some men improve erectile function. Other treatments for erectile dysfunction (alprostadil injections, vacuum devices, penile implants) may also be options.
Radiation Therapy
Radiation therapy may be used as an initial treatment for localized prostate cancer. It may also be used as treatment for cancer that has not been fully removed or has recurred after surgery. In advanced cancer, radiation therapy is used to shrink the size of the tumor and relieve symptoms.
The two main radiation treatments for prostate cancer are:
- External beam radiation
- Brachytherapy (internal radiation)
In some cases, both techniques may be used to treat high-risk patients.
External Beam Radiation
External beam radiation therapy (EBRT) uses a machine that focuses a beam of radiation directly on the tumor. An EBRT treatment lasts a few minutes and is given usually 5 days a week over the course of 7 to 9 weeks. Doctors use imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) to precisely map out where the beams are aimed at the tumor cells.
Newer types of EBRT allow doctors to increase radiation doses while minimizing damage to nearby tissue. Higher radiation doses may reduce the risk for cancer recurrence and improve survival outcomes. Three-dimensional conformal radiation therapy (3D-CRT) uses a computerized program and 3-dimensional image of the prostate to precisely target the tumor. Other techniques related to 3D-CRT include:
Intensity modulated radiation beam therapy (IMRBT)
is an advanced form of 3D-CRT. With IMRBT, the doctor can adjust the doses of the radiation beams so that higher doses go directly to the tumor area while lower doses are delivered to adjacent areas. A variation of IMBRT called image guided radiation therapy (IGRT) uses real-time images of the person's prostate to help deliver more precise doses of radiation.Proton beam radiation therapy
is similar to 3D-CRT but uses a proton beam instead of the x-ray type photon beam used in standard radiation. Proton beams cause less damage than photon beams to the tissues they pass through on the way to the target. However, the machines used to deliver proton beams are extremely expensive and are not widely available. Not all insurance companies pay for this type of radiation therapy.
Fatigue is a common side effect for several months following radiation therapy. Other complications may include:
Gastrointestinal and Bowel Complications
Short-term effects include nausea and loss of appetite. Diarrhea is a very common side effect and can last for the duration of therapy. It is often treated with Lomotil. It usually eventually resolves, but some people may have diarrhea flare-ups for years afterward. Newer 3D-CRT techniques may be less likely to cause diarrhea than standard EBRT.
Urinary Problems
Many people experience a need for frequent urination shortly after radiation therapy, and this urgency persists long-term for some people. Some men experience urinary incontinence (loss of bladder control) but this is less common with radiation than with surgery.
Erectile Dysfunction
Unlike surgery, erectile dysfunction does not usually occur immediately following radiation therapy. However, the risk for this complication increases progressively over a year or more. Drug therapies for erectile dysfunction may help.
Increased Risk for Other Cancers
Men treated with radiation therapy may have increased risks for colorectal or bladder cancer.
Brachytherapy (Internal Radiation)
Brachytherapy is a type of radiation therapy used mainly for men who have early-stage or localized prostate cancer. It can also be used in combination with external beam radiation therapy.
Brachytherapy involves implanting radioactive pellets ("seeds") directly into the prostate. Implants can be permanent or temporary.
- In
permanent brachytherapy
, the pellets are surgically implanted and sealed in place to continue to deliver low-dose radiation for weeks or months. - In
temporary brachytherapy
, the pellets are deposited and held temporarily in place inside of catheters for a treatment session that lasts 5 to 15 minutes. The catheters and pellets are then removed. The person often receives about 3 treatments over the course of 2 days. With temporary brachytherapy, a higher dose of radiation can be used.
Side effects for brachytherapy are similar to those for external beam radiation therapy. Side effects specific to brachytherapy include:
Seed Migration.
In some cases the seeds can move (migrate). If seeds migrate, they often end up in the urethra or bladder and are passed out of the body through urination or ejaculation. (Condoms should be worn for first times of intercourse following seed implantation.) Seeds may potentially migrate through the bloodstream to other parts of the body such as the lungs but this happens very rarely and does not appear to cause any long-term problems.Radiation Exposure.
With permanent brachytherapy, the person can emit small, low-dose amounts of radiation for several weeks. During this time, the person needs to minimize contact with pregnant women or small children, and he will need to wear a condom during sex.PSA Bounce.
It is common for PSA levels to temporarily rise, or "bounce," following seed implantation but this is not a sign of cancer recurrence or cause for concern.
Adjuvant and Salvage Radiation
Radiation may help select people who still have detectable levels of PSA after surgery (generally less than or equal to 2 ng/mL or 2 mcg/L). It may even be useful years after surgery if PSA levels rise.
Depending on timing, radiation after treatment failure is referred to as either:
Adjuvant radiation
is radiation therapy performed within 6 months after radical prostatectomy. One area of controversy is whether to use adjuvant radiation after surgery on people whose PSA levels are very low or undetectable but who have other test results that indicate the cancer is likely to spread. People with adverse findings and low PSA have to weigh the potential complications of radiation therapy against the odds of recurrence without it.Salvage radiation
is radiation therapy given more than 6 months after surgery. Some studies suggest that salvage radiation could be more beneficial than previously thought, even for men with aggressive prostate cancer.
Cryosurgery (Cryoablation)
Cryosurgery is an alternative to standard prostatectomy for men with early-stage, localized prostate cancer who do not want or who are not appropriate candidates for radical prostatectomy. It is also an alternative to radiation therapy.
The goal of cryosurgery is destruction of the entire prostate gland and possibly surrounding tissue. Steel probes are inserted through the skin between the anus and the rectum and guided into the prostate using transrectal ultrasound. Liquid nitrogen is pumped through the probes to freeze all prostate cells, both healthy and cancerous. For success, cryosurgery requires a uniformly frozen area. The dead cells are absorbed and eliminated by the body.
Cryosurgery is typically a 2-hour outpatient procedure, although some people may need to stay in the hospital overnight. Cryosurgery may also be used as a salvage procedure for people who have undergone radiation therapy and have had cancer recurrence detected early. It is generally not helpful for men with very large prostate glands.
Nearly all people experience erectile dysfunction after cryosurgery, and urinary incontinence is also common in men who were first treated with radiation therapy. Other complications of cryosurgery include urinary retention, swelling, and fistula formation. Incontinence and fistulas are more likely to occur when cryosurgery is used as a salvage procedure than when it is used as a primary procedure.
This therapy is still considered experimental by some doctors, and there are no long-term data to compare its effectiveness with standard prostatectomy. For this reason, cryosurgery is generally not considered as a first-line initial treatment.
Hormone Therapy and Chemotherapy
Androgen Deprivation Therapy (Hormone Therapy)
Androgen deprivation therapy (also called androgen suppression therapy or hormone therapy) uses treatments to reduce levels of male hormones such as testosterone, which stimulate the growth of prostate cells. Androgen deprivation therapy suppresses or blocks testosterone by either:
Surgical castration.
Bilateral orchiectomy is surgical removal of the testicles. This is an irreversible procedure that permanently stops testosterone production.Chemical castration.
Drugs such as LH-RH agonists or anti-androgens interfere with testosterone production or activity. Once the drug is stopped, testosterone production and action resumes.
Androgen deprivation therapy is not usually recommended as an initial therapy for early-stage prostate cancer. Studies indicate that it does not improve survival for most men with localized prostate cancer. It is mainly used for:
- Advanced (metastatic) cancer that has spread beyond the prostate gland
- Cancer that has failed to respond to surgery or radiation
- Cancer that has recurred
Androgen deprivation therapy may also be used:
- Before radiation or surgery to help shrink tumors
- Along with radiation therapy for cancer that is likely to recur
- Before, during, and after radiation therapy for locally advanced prostate cancer
- As palliative therapy to help ease cancer symptoms, or to prevent symptoms of progressing cancer
Surgical Castration (Orchiectomy)
Bilateral orchiectomy is the surgical removal of both testicles (surgical castration). It is the single most effective method of reducing androgen hormones but its effects are permanent. Orchiectomy plus radical prostatectomy may delay progression in people with cancers that have spread only to the pelvic lymph nodes.
Men who have orchiectomy have reduced sexual function and desire. People do not experience a reversal of sex characteristics and the voice does not change. Like all androgen deprivation therapies, orchiectomy increases the risk for osteoporosis.
Chemical Castration (Hormone Drug Therapy)
LH-RH Agonists and Antagonists
The main drugs used for suppressing androgens are called luteinizing hormone-releasing hormones (LH-RH) agonists. They are also known as gonadotropin-releasing hormone (GnRH) agonists or LH-RH analogs. These drugs block the pituitary gland from producing hormones that stimulate testosterone production.
LH-RH agonists include leuprolide (Lupron, Eligard, Viadur, generic), goserelin (Zoladex), triptorelin (Trelstar), and histrelin (Vantas). These drugs are given as injections or as implants placed under the skin. LH-RH agonists produce a testosterone surge ("flare") in the first week, which may intensify symptoms including bone pain if the cancer has spread to the bones. After this phase, testosterone levels drop to near zero. LHRH agonists can also cause PSA levels to rise temporarily.
LH-RH antagonists work similarly to LH-RH agonists but do not cause a testosterone surge. The FDA has approved the LH-RH antagonist drugs degarelix (Firmagon) and relugolix (Orgovyx) for treatment of advanced prostate cancer.
Common side effects of these drugs include hot flashes, fatigue, testicle shrinkage, and breast enlargement. These drugs may increase blood sugar levels and the risk of developing diabetes. They may also increase risk for heart attack, stroke, and sudden death.
Anti-Androgens
Anti-androgens are drugs used to block the effects of testosterone. Anti-androgens are not typically used by themselves. They are often added when another hormone therapy stops working. They are generally used in combination with LH-RH agonists. This type of treatment is called combined androgen blockade.
The anti-androgen drugs used for prostate cancer treatment include flutamide (Eulexin, generic), nilutamide (Nilandron), both of which are rarely used today. The more commonly used anti-androgen drugs are enzalutamide (Xtandi), apalutamide (Erleada), and bicalutamide (Casodex, generic). They are taken as daily pills. When taken with an LH-RH agonist, hot flashes and breast tenderness is intensified.
Side Effects
All types of hormone drug therapy can help delay cancer growth but this treatment can cause significant side effects including:
- Hot flashes, which may go away over time.
- Sexual dysfunction (erectile dysfunction) and loss of sexual drive (low libido).
- Osteoporosis, the loss of bone density. Medications such as denosumab (Prolia) and zoledronic acid (Zometa) help increase bone mass and prevent bone fractures in men receiving hormone therapy.
- Decrease in HDL (good cholesterol) levels.
- Loss of muscle mass.
- Weight gain.
- Decreased mental alertness.
- Fatigue and depression.
- Swelling and tenderness of the breasts (gynecomastia).
- Anemia (low red blood cell count).
In addition, there is growing evidence that androgen deprivation drug therapy increases the risks for heart attack, stroke, and diabetes. These drugs increase body weight, which can lead to decreased insulin sensitivity and harmful changes in cholesterol levels.
Guidelines recommend that men who receive androgen deprivation therapy should have regular follow-up exams with their primary care doctors within 3 to 6 months after starting therapy. The doctor should monitor the patient's blood pressure and perform blood sugar (glucose) and cholesterol (lipid) laboratory tests at least once a year.
Continuous Versus Intermittent Therapy
When prescribing hormone therapy drugs, some doctors recommend periodically stopping and restarting treatment (intermittent therapy). This approach appears to help reduce erectile dysfunction, hot flashes, and other hormone therapy side effects that impair quality of life.
However, it is not clear if intermittent therapy works as well as continuous therapy for prostate cancer treatment. Some studies indicate that continuous therapy is more effective or that intermittent therapy should only be used for select types of prostate cancer. Other research suggests that intermittent androgen deprivation is as effective as continuous therapy. More research is needed.
Treatments for Castration-Resistant Cancer
Hormone therapy is initially very effective, but after several years the cancer often starts growing again. Prostate cancer that no longer responds to hormonal treatment is called castration-resistant. (It was formerly called hormone-resistant or hormone-refractory cancer.) When castration-resistant prostate cancer advances and spreads throughout the body it is called metastatic castration-resistant prostate cancer (mCRPC). There are several treatment options, including new types of drugs. Most of these therapies prolong survival by a few months.
The American Society of Clinical Oncology (ASCO) has treatment guidelines for mCRPC that take into consideration both the quality of life and survival benefits of various therapies. The ASCO guidelines recommend that for patients with mCRPC, hormone therapy should be continued indefinitely. In addition, men should be offered one of three treatments: abiraterone (Zytiga) plus prednisone, enzalutamide (Xtandi), or radium-223 (Xofigo). Chemotherapy or other select therapies may be options for certain patients. Palliative care (pain-relieving measures) should be offered to all people.
Abiraterone (Zytiga)
Abiraterone (Zytiga) is a drug that is used for patients with metastatic castration-resistant prostate cancer and for people with high risk castrate-sensitive metastatic cancer. It blocks an enzyme called CYP17. The drug is taken as once-daily pills along with prednisone. Abiraterone is also approved for use for metastatic cancer prior to chemotherapy. Side effects may include joint swelling, fluid build-up in legs and feet, muscle discomfort, hot flashes, high blood pressure, and low levels of potassium in the blood.
Non-steroidal Antiandrogens
Non-steroidal antiandrogens are drugs that block the androgen receptor and thus inhibit the effects of androgens such as testosterone.
Enzalutamide (Xtandi) is an anti-androgen drug for men with castrate-resistant prostate cancer that has or has not spread (metastasized). Enzalutamide is taken as a daily pill. Unlike abiraterone, it does not need to be taken along with prednisone. Its side effects are similar to abiraterone except that enzalutamide may increase the risk for seizures.
Apalutamide (Erleada) is another nonsteroidal anti-androgen recently approved by the FDA for castration-resistant prostate cancer that has not spread (metastasized).
Darolutamide (Nubeqa) is used to treat castration-resistant prostate cancer that has not spread. Used together with castration, darolutamide has side effects including tiredness, rash, and arm and leg pain.
All these non-steroidal antiandrogens are used together with surgical or chemical castration.
Radium-223 (Xofigo)
Radium-223 injection (Xofigo) is approved for treatment of metastatic castration-resistant prostate cancer that has spread to the bones but not to other organs. Xofigo is a radioactive medicine that binds to the mineral in bones. It targets and delivers radiation directly to bone tumors without damaging surrounding healthy tissue. In clinical trials, men treated with Xofigo lived about 3 months longer than those who did not receive the drug.
Chemotherapy
The main first-line chemotherapy treatment for mCRPC is docetaxel (Taxotere) combined with the corticosteroid prednisone. Recent evidence suggests that some people with metastatic prostate cancer may benefit more from docetaxel chemotherapy as first-line therapy when combined with androgen deprivation therapy than from androgen therapy alone.
Cabazitaxel (Jevtana) is a newer chemotherapy drug approved for people whose condition has worsened during or after treatment with docetaxel. It is also used in combination with prednisone. Mitoxantrone (Novantrone, generic) plus prednisone is a third option for chemotherapy but it appears to have limited clinical benefit.
Chemotherapy regimens may help extend survival time by several months. Side effects can be serious and may include gastrointestinal problems (nausea, vomiting, or diarrhea), fatigue, low blood cell counts, and increased risk for blood clots. Men who are considering chemotherapy should be sure to discuss with their doctors all potential side effects.
Sipuleucel-T (Provenge)
Sipuleucel-T (Provenge) is a cancer "vaccine" approved for select men with advanced prostate cancer. Unlike typical vaccines, it does not prevent disease. Instead, it uses the patient's own immune system to fight the cancer. Each vaccine is individually manufactured by collecting the person's own immune cells from peripheral blood. The cells are then exposed to a special type of protein and infused back into the patient. The American Society of Clinical Oncology (ASCO) recommends this treatment only for men with mCRPC who have few or no symptoms of cancer.
Provenge extends survival time by an average of 4 months. Quality of life benefits are uncertain. In studies, side effects ranged from mild (chills, fatigue, fever, nausea, joint, and muscle aches) to severe (stroke). Provenge is very expensive, but some insurers, offer coverage for it.
PARP Inhibitors
PARP inhibitors are a group of drugs that inhibit a type of enzyme necessary to repair damaged DNA inside cells. Some cancers, including prostate cancer, may harbor mutations that cause the tumors to be sensitive to the effects of PARP inhibitors. Two such drugs are currently approved for the treatment of prostate cancer. Olaparib (Lynparza) is approved for men with mCRPC who have previously received abiraterone or enzalutamide and who have been found to have the genetic alterations suggesting a benefit from treatment with a PARP inhibitor. Rucaparib (Rubraca) is another PARP inhibitor approved for prostate cancer treatment in men with genetic mutations who have already received chemotherapy.
Resources
- National Cancer Institute -- www.cancer.gov
- American Cancer Society -- www.cancer.org
- Cancer.Net -- www.cancer.net
- Prostate Cancer Foundation -- www.pcf.org
- Urology Care Foundation -- www.urologyhealth.org
- Us Too! Prostate Cancer Education and Support -- www.ustoo.org
- Find clinical trials -- www.cancer.gov/about-cancer/treatment/clinical-trials
References
Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet. 2016;387(10013):70-82. PMID: 26074382 pubmed.ncbi.nlm.nih.gov/26074382/.
Barocas DA, Mallin K, Graves AJ, et al. Effect of the USPSTF Grade D recommendation against screening for prostate cancer on incident prostate cancer diagnoses in the United States. J Urol. 2015;194(6):1587-1593. PMID: 26087383 pubmed.ncbi.nlm.nih.gov/26087383/.
Daskivich TJ, Fan KH, Koyama T, et al. Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S. population-based cohort of men with prostate cancer. Ann Intern Med. 2013;158(10):709-717. PMID: 23689764 pubmed.ncbi.nlm.nih.gov/23689764/.
D'Amico AV, Chen MH, Renshaw A, Loffredo M, Kantoff PW. Long-term follow-up of a randomized trial of radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2015;314(12):1291-1293. PMID: 26393854 pubmed.ncbi.nlm.nih.gov/26393854/.
Eggener S. Hormonal therapy for prostate cancer. In: Partin AW, Dmochowski RR, Kavoussi LR, Peters CA, eds. Campbell-Walsh-Wein Urology. 12th ed. Philadelphia, PA: Elsevier; 2021: chap. 161.
Fenton JJ, Weyrich MS, Durbin S, et al. Prostate-specific antigen-based screening for prostate cancer: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(18):1914-1931. PMID: 29801018 pubmed.ncbi.nlm.nih.gov/29801018/.
Freedland SJ, Rumble RB, Finelli A, et al. Adjuvant and salvage radiotherapy after prostatectomy: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2014;32(34):3892-3898. PMID: 25366677 pubmed.ncbi.nlm.nih.gov/25366677/.
Klotz L. Active surveillance of prostate cancer. In: Partin AW, Dmochowski RR, Kavoussi LR, Peters CA, eds. Campbell-Walsh-Wein Urology. 12th ed. Philadelphia, PA: Elsevier; 2021: chap. 154.
Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317(24):2532-2542. PMID: 28655021 pubmed.ncbi.nlm.nih.gov/28655021/.
Lowrance WT, Breau RH, Chou R, Chapin BF, Crispino T, Dreicer R, Jarrard DF, Kibel AS, Morgan TM, Morgans AK, Oh WK, Resnick MJ, Zietman AL, Cookson MS. Advanced prostate cancer: AUA/ASTRO/SUO Guideline PART I. J Urol. 2021;205(1):14-21. PMID: 32960679 pubmed.ncbi.nlm.nih.gov/32960679/.
Lowrance WT, Breau RH, Chou R, Chapin BF, Crispino T, Dreicer R, Jarrard DF, Kibel AS, Morgan TM, Morgans AK, Oh WK, Resnick MJ, Zietman AL, Cookson MS. Advanced prostate cancer: AUA/ASTRO/SUO Guideline PART II. J Urol. 2021;205(1):22-29. PMID: 32960678 pubmed.ncbi.nlm.nih.gov/32960678/.
PDQ Integrative, Alternative, and Complementary Therapies Editorial Board. Prostate cancer, nutrition, and dietary supplements (PDQ®): Health Professional Version. PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. 2021 Mar 22. PMID: 26389500 pubmed.ncbi.nlm.nih.gov/26389500/.
PDQ Adult Treatment Editorial Board. Prostate cancer treatment (PDQ®): Health Professional Version. PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. 2021 Feb 12. PMID: 26389471 pubmed.ncbi.nlm.nih.gov/26389471/.
PDQ Screening and Prevention Editorial Board. Prostate Cancer Screening (PDQ®): Health Professional Version. 2021 Mar 18. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. Available from: www.ncbi.nlm.nih.gov/books/NBK65945/.
Qaseem A, Barry MJ, Denberg TD, Owens DK, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013;158(10):761-769. PMID: 23567643 pubmed.ncbi.nlm.nih.gov/23567643/.
Resnick MJ, Lacchetti C, Penson DF; American Society of Clinical Oncology. Prostate cancer survivorship care guidelines: American Society of Clinical Oncology practice guideline endorsement. J Oncol Pract. 2015;11(3):e445-e449. PMID: 25829527 pubmed.ncbi.nlm.nih.gov/25829527/.
Salami SS, Palapattu GS, Partin AW, Morgan TM. In: Partin AW, Dmochowski RR, Kavoussi LR, Peters CA, eds. Campbell-Walsh-Wein Urology. 12th ed. Philadelphia, PA: Elsevier; 2021: chap. 149.
Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657. PMID: 29412780 pubmed.ncbi.nlm.nih.gov/29412780/.
Schaeffer E, Srinivas S, Antonarakis ES, et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J Natl Compr Canc Netw. 2021;19(2):134-143. PMID: 33545689 pubmed.ncbi.nlm.nih.gov/33545689/.
Stephenson AJ, Abouassaly R, Klein EA. Epidemiology, etiology, and prevention of prostate cancer. In: Partin AW, Dmochowski RR, Kavoussi LR, Peters CA, eds. Campbell-Walsh-Wein Urology. 12th ed. Philadelphia, PA: Elsevier; 2021: chap. 148.
Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479-499. PMID: 30691365 pubmed.ncbi.nlm.nih.gov/30691365/.
US Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(18):1901-1913. PMID: 29801017 pubmed.ncbi.nlm.nih.gov/29801017/.
Virgo KS, Rumble RB, de Wit R, et al. Initial management of noncastrate advanced, recurrent, or metastatic prostate cancer: ASCO guideline update. J Clin Oncol. 2021;39(11):1274-1305. PMID: 33497248 pubmed.ncbi.nlm.nih.gov/33497248/.
|
Review Date:
5/29/2021 Reviewed By: Kelly L. Stratton, MD, FACS, Associate Professor, Department of Urology, University of Oklahoma Health Sciences Center, Oklahoma City, OK. Also reviewed by David Zieve, MD, MHA, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team. |